Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly Italian population
- PMID: 29425079
- PMCID: PMC6037461
- DOI: 10.1080/21645515.2018.1438792
Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly Italian population
Abstract
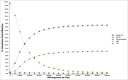
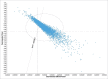
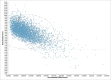
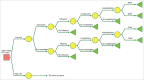
ABSRACT In the perspective of reaching at least 75% influenza vaccination coverage in the elderly and substantial budget constraints, Italian decision makers are facing important challenges in determining an optimal immunization strategy for this growing and particularly vulnerable population. Four different influenza vaccines are currently available for Italian older adults aged 65 years or above, namely trivalent inactivated vaccines (TIVs), MF59-adjuvanted TIV (MF59-TIV), intradermal TIV (ID-TIV) and quadrivalent inactivated vaccines (QIVs). The present study is the first to compare the cost-effectiveness profiles of virtually all possible public health strategies, including the aforementioned four vaccine formulations as well non-vaccination. For this purpose, a decision tree model was built ex novo; the analysis was conducted from the third-payer perspective in the timeframe of one year. All available vaccines were cost-effective compared with non-vaccination. However, MF59-TIV had the most favorable economic profile in the Italian elderly population. Indeed, compared with non-vaccination, it was deemed highly cost-effective with an incremental cost-effectiveness ratio (ICER) of €10,750 per quality-adjusted life year (QALY). The ICER was much lower (€4,527/QALY) when MF59-TIV was directly compared with TIV. ID-TIV and QIV were dominated by MF59-TIV as the former comparators were associated with greater total costs and lower health benefits. Both deterministic and probabilistic sensitivity analyses confirmed robustness of the base case results. From the economic perspective, MF59-TIV should be considered as a preferential choice for Italian older adults aged 65 years or above.
Keywords: Influenza; Italy; adjuvanted influenza vaccine; cost-effectiveness; costs; economic evaluation; elderly; vaccine.
Figures
References
-
- Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, Bresee JS. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2016–17 influenza season. MMWR Recomm Rep. 2016;65:1–54. doi:10.15585/mmwr.rr6505a1. PMID:27560619. - DOI - PubMed
-
- European Centre for Disease Prevention and Control Seasonal influenza vaccination and antiviral use in Europe – Overview of vaccination recommendations and coverage rates in the EU Member States for the 2013–14 and 2014–15 influenza seasons. Stockholm: ECDC. 2016. Available from: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publicat... (Accessed 12October2017) 2016.
-
- Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87:461–76. PMID:23210147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
