Determinants of Optogenetic Cortical Spreading Depolarizations
- PMID: 29425263
- PMCID: PMC6373833
- DOI: 10.1093/cercor/bhy021
Determinants of Optogenetic Cortical Spreading Depolarizations
Abstract
Cortical spreading depolarization (SD) is the electrophysiological event underlying migraine aura, and a critical contributor to secondary damage after brain injury. Experimental models of SD have been used for decades in migraine and brain injury research; however, they are highly invasive and often cause primary tissue injury, diminishing their translational value. Here we present a non-invasive method to trigger SDs using light-induced depolarization in transgenic mice expressing channelrhodopsin-2 in neurons (Thy1-ChR2-YFP). Focal illumination (470 nm, 1-10 mW) through intact skull using an optical fiber evokes power-dependent steady extracellular potential shifts and local elevations of extracellular [K+] that culminate in an SD when power exceeds a threshold. Using the model, we show that homozygous mice are significantly more susceptible to SD (i.e., lower light thresholds) than heterozygous ChR2 mice. Moreover, we show SD susceptibility differs significantly among cortical divisions (motor, whisker barrel, sensory, visual, in decreasing order of susceptibility), which correlates with relative channelrhodopsin-2 expression. Furthermore, the NMDA receptor antagonist MK-801 blocks the transition to SD without diminishing extracellular potential shifts. Altogether, our data show that the optogenetic SD model is highly suitable for examining physiological or pharmacological modulation of SD in acute and longitudinal studies.
Figures
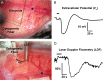

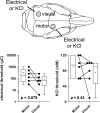

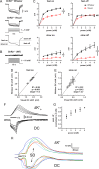

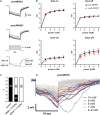

References
-
- Ayata C. 2009. Spreading depression: from serendipity to targeted therapy in migraine prophylaxis. Cephalalgia. 29:1095–1114. - PubMed
-
- Ayata C. 2010. Cortical spreading depression triggers migraine attack: pro. Headache. 50:725–730. - PubMed
-
- Ayata C. 2013. Pearls and pitfalls in experimental models of spreading depression. Cephalalgia. 33:604–613. - PubMed
-
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. 2006. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 59:652–661. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

