Associations Between Serum Bone Biomarkers in Early Breast Cancer and Development of Bone Metastasis: Results From the AZURE (BIG01/04) Trial
- PMID: 29425304
- PMCID: PMC6093369
- DOI: 10.1093/jnci/djx280
Associations Between Serum Bone Biomarkers in Early Breast Cancer and Development of Bone Metastasis: Results From the AZURE (BIG01/04) Trial
Abstract
Background: Adjuvant therapies can prevent/delay bone metastasis development in breast cancer. We investigated whether serum bone turnover markers in early disease have clinical utility in identifying patients with a high risk of developing bone metastasis.
Methods: Markers of bone formation (N-terminal propeptide of type-1 collagen [P1NP]) and bone resorption (C-telopeptide of type-1 collagen [CTX], pyridinoline cross-linked carboxy-terminal telopeptide of type-1 collagen [1-CTP]) were measured in baseline (pretreatment blood samples from 872 patients from a large randomized trial of adjuvant zoledronic acid (AZURE-ISRCTN79831382) in early breast cancer. Cox proportional hazards regression and cumulative incidence functions (adjusted for factors having a statistically significant effect on outcome) were used to investigate prognostic and predictive associations between recurrence events, bone marker levels, and clinical variables. All statistical tests were two-sided.
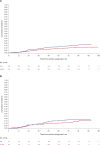
Results: When considered as continuous variables (log transformed), P1NP, CTX, and 1-CTP were each prognostic for future bone recurrence at any time (P = .006, P = .009, P = .008, respectively). Harrell's c-indices were a P1NP of 0.57 (95% confidence interval [CI] = 0.51 to 0.63), CTX of 0.57 (95% CI = 0.51 to 0.62), and 1-CTP of 0.57 (95% CI = 0.52 to 0.63). In categorical analyses based on the normal range, high baseline P1NP (>70 ng/mL) and CTX (>0.299 ng/mL), but not 1-CTP (>4.2 ng/mL), were also prognostic for future bone recurrence (P = .03, P = .03, P = .10, respectively). None of the markers were prognostic for overall distant recurrence; that is, they were bone metastasis specific, and none of the markers were predictive of treatment benefit from zoledronic acid.
Conclusions: Serum P1NP, CTX, and 1-CTP are clinically useful, easily measured markers that show good prognostic ability (though low-to-moderate discrimination) for bone-specific recurrence and are worthy of further study.
Figures


References
-
- US Breast Cancer Statistics. 2017. http://www.breastcancer.org/symptoms/understand_bc/statistics.
-
- Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): A multicentre, placebo-controlled, randomized trial. Lancet Oncol. 2012;13:734–742.10.1016/S1470-2045(12)70226-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

