Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice
- PMID: 29426327
- PMCID: PMC5810193
- DOI: 10.1186/s12974-017-1052-x
Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice
Abstract
Background: Porphyromonas gingivalis lipopolysaccharide (P. gingivalis-LPS) is one of the major pathogenic factors of chronic periodontitis (CP). Few reports on the correlation between P. gingivalis-LPS and cognitive function exist. Thus, the present study aimed to investigate the effects of P. gingivalis-LPS on cognitive function and the associated underlying mechanism in C57BL/6 mice.
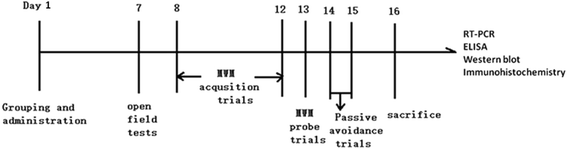
Methods: The C57BL/6 mice were injected with P. gingivalis-LPS (5 mg kg-1) either with or without Toll-like receptor 4 (TLR4) inhibitor (TAK-242, 5 mg kg-1). After 7 days, behavioral alterations were assessed with the open field test (OFT), Morris water maze (MWM) test, and passive avoidance test (PAT). The activation of astrocytes and microglia in the cerebral cortex and hippocampus of mice was observed by immunohistochemistry. The expression of inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8), TLRs (TLR2, TLR3, and TLR4), and CD14 and the activation of the NF-κB signaling pathway (IRAK1, p65, and p-p65) in the cerebral cortex of the mice were evaluated by RT-PCR, ELISA, and western blot.
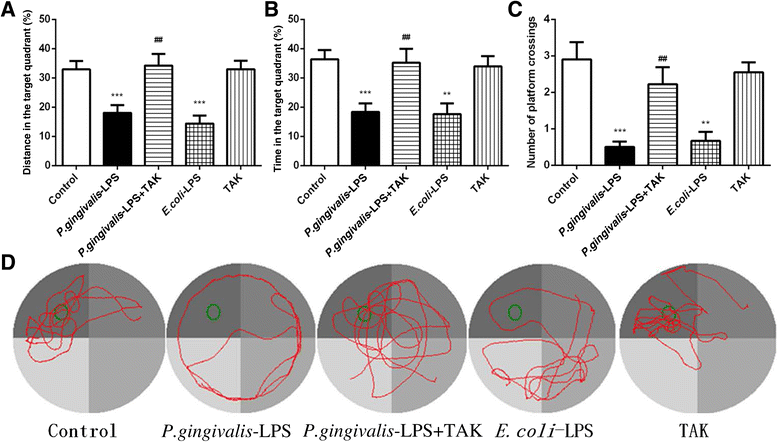
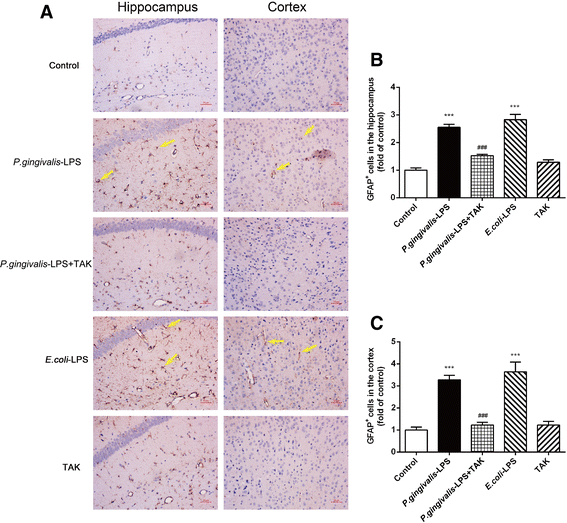
Results: The OFT showed that P. gingivalis-LPS did not affect the initiative and activity of mice. Administration of P. gingivalis-LPS significantly impaired spatial learning and memory during the MWM test and attenuated the ability of passive avoidance learning during the PAT. Both astrocytes and microglia were activated in the cortex and hippocampus. The messenger RNA (mRNA) and protein expression of inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) was upregulated by P. gingivalis-LPS in the cortex. In addition, the TLR4/NF-κB signaling pathway was activated (TLR4, CD14, IRAK1, and p-p65). These effects were effectively alleviated by TAK-242.
Conclusions: Administration of P. gingivalis-LPS can lead to learning and memory impairment in C57BL/6 mice. This impairment is mediated by activation of the TLR4 signaling pathway. Our study suggests that P. gingivalis-LPS-induced neuroinflammation plays an important role in cognitive impairment. It also reveals that endotoxins of periodontal pathogens could represent a risk factor for cognitive disorders.
Keywords: Cognition; Lipopolysaccharide; Neuroinflammation; Porphyromonas gingivalis; TLR4.
Conflict of interest statement
Ethics approval
The animal experiments were approved by the Animal Care and Welfare Committee of Shanghai Jiao Tong University School of Medicine (approval ID: A-2016-032).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures








Similar articles
-
Lipopolysaccharide Preparation Derived From Porphyromonas gingivalis Induces a Weaker Immuno-Inflammatory Response in BV-2 Microglial Cells Than Escherichia coli by Differentially Activating TLR2/4-Mediated NF-κB/STAT3 Signaling Pathways.Front Cell Infect Microbiol. 2021 Mar 18;11:606986. doi: 10.3389/fcimb.2021.606986. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33816329 Free PMC article.
-
Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway.CNS Neurosci Ther. 2019 May;25(5):575-590. doi: 10.1111/cns.13086. Epub 2019 Jan 24. CNS Neurosci Ther. 2019. PMID: 30676698 Free PMC article.
-
Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-κB Signaling Pathway.Biomed Res Int. 2019 Aug 20;2019:6282635. doi: 10.1155/2019/6282635. eCollection 2019. Biomed Res Int. 2019. PMID: 31531360 Free PMC article.
-
Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors.Crit Rev Oral Biol Med. 2002;13(2):132-42. doi: 10.1177/154411130201300204. Crit Rev Oral Biol Med. 2002. PMID: 12097356 Review.
-
Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway.J Neuroinflammation. 2019 Jul 18;16(1):148. doi: 10.1186/s12974-019-1538-9. J Neuroinflammation. 2019. PMID: 31319868 Free PMC article. Review.
Cited by
-
Oral Microbiome Dysbiosis as a Risk Factor for Stroke: A Comprehensive Review.Microorganisms. 2024 Aug 22;12(8):1732. doi: 10.3390/microorganisms12081732. Microorganisms. 2024. PMID: 39203574 Free PMC article. Review.
-
The oral-brain axis: can periodontal pathogens trigger the onset and progression of Alzheimer's disease?Front Microbiol. 2024 Feb 1;15:1358179. doi: 10.3389/fmicb.2024.1358179. eCollection 2024. Front Microbiol. 2024. PMID: 38362505 Free PMC article. Review.
-
Possible Role of Porphyromonas gingivalis in the Regulation of E2F1, CDK11, and iNOS Gene Expression in Neuronal Cell Cycle: A Preliminary Study.J Int Soc Prev Community Dent. 2021 Sep 21;11(5):582-587. doi: 10.4103/jispcd.JISPCD_108_21. eCollection 2021 Sep-Oct. J Int Soc Prev Community Dent. 2021. PMID: 34760804 Free PMC article.
-
Porphyromonas gingivalis-Induced Neuroinflammation in Alzheimer's Disease.Front Neurosci. 2021 Oct 14;15:691016. doi: 10.3389/fnins.2021.691016. eCollection 2021. Front Neurosci. 2021. PMID: 34720846 Free PMC article. Review.
-
Lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli: Differential and interactive effects on novelty-induced hyperlocomotion, blood cytokine levels and TLR4-related processes.PLoS One. 2024 Jun 10;19(6):e0292830. doi: 10.1371/journal.pone.0292830. eCollection 2024. PLoS One. 2024. PMID: 38857232 Free PMC article.
References
-
- Zhou X, Han J, Liu Z, et al. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41:564–572. doi: 10.1111/jcpe.12247. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

