Nursing home care educational intervention for family caregivers of older adults post stroke (SHARE): study protocol for a randomised trial
- PMID: 29426361
- PMCID: PMC5807750
- DOI: 10.1186/s13063-018-2454-5
Nursing home care educational intervention for family caregivers of older adults post stroke (SHARE): study protocol for a randomised trial
Abstract
Background: Family caregivers of aged stroke survivors face challenging difficulties such as the lack of support and the knowledge and skills to practice home care. These aspects negatively influence the caregivers' burden and quality of life, the use of health services, and hospital readmissions of the stroke survivor. The aim of this research is to describe an educational intervention focused on family caregivers of stroke survivors for the development of home care in the south of Brazil.
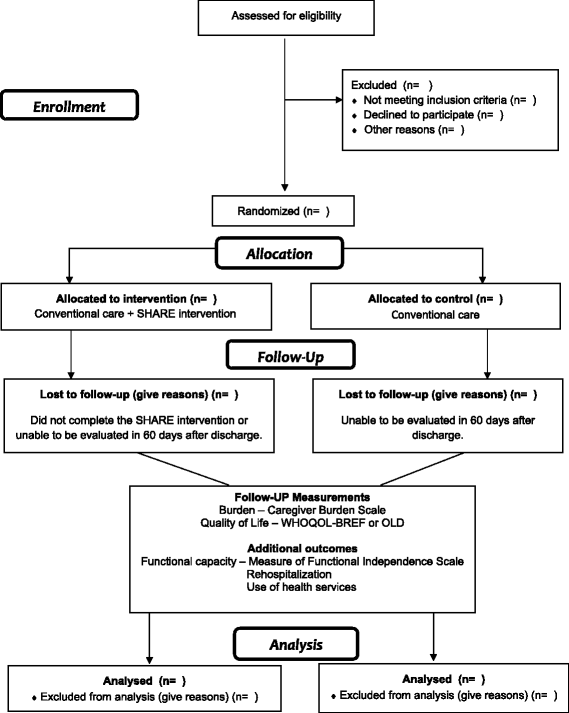
Methods: A randomized clinical trial with 48 family caregivers of stroke survivors will be recruited and divided into two groups: 24 in the intervention group and 24 in the control group. The intervention will consist of the systematic follow-up by nurses who will perform three home visits over a period of 1 month. The control group will not receive the visits and will have the usual care guidelines of the health services. Primary outcomes: burden and quality of life of the caregiver.
Secondary outcomes: functional capacity and readmissions of the stroke survivors; the use of health services of the stroke survivors and their family caregivers. Outcomes will be measured 2 months after discharge. The project was approved in April 2016.
Discussion: This research offers information for conducting educational intervention with family caregivers of stroke survivors, presenting knowledge so that nurses can structure and plan the actions aimed at the education of the family caregiver. It is expected that the educational intervention will contribute to reducing caregiver burden and improving their quality of life, as well as avoiding readmissions and inadequate use of health services by stroke survivors.
Trial registration: ClinicalTrials.gov, ID: NCT02807012 . Registered on 3 June 2016. Name: Nursing Home Care Intervention Post Stroke (SHARE).
Keywords: Aged; Caregivers; Home care services; Nursing; Randomized controlled trial; Stroke.
Conflict of interest statement
Ethics approval and consent to participate
This study presented all criteria to be approval by Ethical Research Committee of Hospital de Clinicas de Porto Alegre (HCPA), number: 16-0181. The consent is appropriate and has all the criteria required by HCPA. All stroke survivors and their caregivers will sign the informed consent, which includes the ethical aspects of confidentiality and voluntary participation.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization (WHO). The atlas of heart disease and stroke. 2016. http://www.who.int/cardiovascular_diseases/resources/atlas/en/. Accessed 2 Jan 2017.
-
- Brasil. Ministério da Saúde. Indicadores de Saúde e Pactuação. DATASUS. 2010. http://www2.datasus.gov.br/DATASUS/index.php?area=0201&VObj=http://tabne.... Accessed 2 Feb 2017.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous