Cellular imaging by targeted assembly of hot-spot SERS and photoacoustic nanoprobes using split-fluorescent protein scaffolds
- PMID: 29426856
- PMCID: PMC5807522
- DOI: 10.1038/s41467-018-03046-w
Cellular imaging by targeted assembly of hot-spot SERS and photoacoustic nanoprobes using split-fluorescent protein scaffolds
Abstract
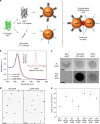
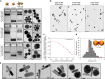
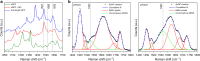
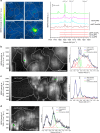
The in cellulo assembly of plasmonic nanomaterials into photo-responsive probes is of great interest for many bioimaging and nanophotonic applications but remains challenging with traditional nucleic acid scaffolds-based bottom-up methods. Here, we address this quandary using split-fluorescent protein (FP) fragments as molecular glue and switchable Raman reporters to assemble gold or silver plasmonic nanoparticles (NPs) into photonic clusters directly in live cells. When targeted to diffusing surface biomarkers in cancer cells, the NPs self-assemble into surface-enhanced Raman-scattering (SERS) nanoclusters having hot spots homogenously seeded by the reconstruction of full-length FPs. Within plasmonic hot spots, autocatalytic activation of the FP chromophore and near-field amplification of its Raman fingerprints enable selective and sensitive SERS imaging of targeted cells. This FP-driven assembly of metal colloids also yields enhanced photoacoustic signals, allowing the hybrid FP/NP nanoclusters to serve as contrast agents for multimodal SERS and photoacoustic microscopy with single-cell sensitivity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

