Open-Label Placebo Treatment for Cancer-Related Fatigue: A Randomized-Controlled Clinical Trial
- PMID: 29426869
- PMCID: PMC5807541
- DOI: 10.1038/s41598-018-20993-y
Open-Label Placebo Treatment for Cancer-Related Fatigue: A Randomized-Controlled Clinical Trial
Abstract
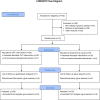
The purpose of this 21-day assessor blinded, randomized-controlled trial was to compare an open-label placebo (OLP) to treatment as usual (TAU) for cancer survivors with fatigue. This was followed by an exploratory 21-day study in which TAU participants received OLPs while OLP participants in the main study were followed after discontinuing placebos. Cancer survivors (N = 74) who completed cancer treatment 6 months to 10 years prior to enrollment reporting at least moderate fatigue (i.e., ≥4 on a 0-10 scale) were randomized to OLP or TAU. Those randomized to OLP took 2 placebo pills twice a day for 21 days. Compared to those randomized to TAU, OLP participants reported a 29% improvement in fatigue severity (average difference in the mean change scores (MD) 12.47, 95% CI 3.32, 21.61; P = 0.008), medium effect (d = 0.63), and a 39% improvement in fatigue-disrupted quality of life (MD = 11.76, 95% CI 4.65, 18.86; P = 0.002), a large effect (d = 0.76). TAU participants who elected to try OLP for 21-days after the main study reported reductions in fatigue of a similar magnitude for fatigue severity and fatigue-disrupted quality of life (23% and 35%, respectively). OLP may reduce fatigue symptom severity and fatigue-related quality of life disruption in cancer survivors.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Clinical practice guidelines in oncology: Cancer-related fatigue. NCCN Guidelines, http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf (2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

