Involvement of orexin neurons in fasting- and central adenosine-induced hypothermia
- PMID: 29426934
- PMCID: PMC5807529
- DOI: 10.1038/s41598-018-21252-w
Involvement of orexin neurons in fasting- and central adenosine-induced hypothermia
Abstract
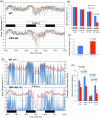
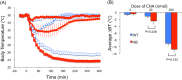
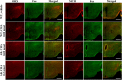
We examined whether orexin neurons might play a protective role against fasting- and adenosine-induced hypothermia. We first measured body temperature (BT) in orexin neuron-ablated (ORX-AB) mice and wild-type (WT) controls during 24 hours of fasting. As expected, the magnitude of BT drop and the length of time suffering from hypothermia were greater in ORX-AB mice than in WT mice. Orexin neurons were active just before onset of hypothermia and during the recovery period as revealed by calcium imaging in vivo using G-CaMP. We next examined adenosine-induced hypothermia via an intracerebroventricular administration of an adenosine A1 receptor agonist, N6-cyclohexyladenosine (CHA), which induced hypothermia in both ORX-AB and WT mice. The dose of CHA required to initiate a hypothermic response in ORX-AB mice was more than 10 times larger than the dose for WT mice. Once hypothermia was established, the recovery was seemingly slower in ORX-AB mice. Activation of orexin neurons during the recovery phase was confirmed by immunohistochemistry for c-Fos. We propose that orexin neurons play dual roles (enhancer in the induction phase and compensator during the recovery phase) in adenosine-induced hypothermia and a protective/compensatory role in fasting-induced hypothermia.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hudson JW, Scott IM. Daily torpor in the laboratory mouse, mus musculus var. albino. Physiol Biochem Zoology. 1979;52:205–218.
-
- Aslami H, Juffermans N. Induction of a hypometabolic state during critical illness - a new concept in the ICU? Neth J Med. 2010;68:190–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

