Two microcephaly-associated novel missense mutations in CASK specifically disrupt the CASK-neurexin interaction
- PMID: 29426960
- PMCID: PMC6391276
- DOI: 10.1007/s00439-018-1874-3
Two microcephaly-associated novel missense mutations in CASK specifically disrupt the CASK-neurexin interaction
Abstract
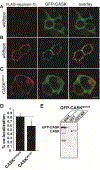
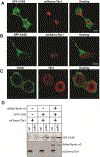
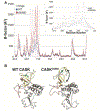
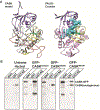
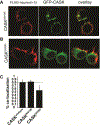
Deletion and truncation mutations in the X-linked gene CASK are associated with severe intellectual disability (ID), microcephaly and pontine and cerebellar hypoplasia in girls (MICPCH). The molecular origin of CASK-linked MICPCH is presumed to be due to disruption of the CASK-Tbr-1 interaction. This hypothesis, however, has not been directly tested. Missense variants in CASK are typically asymptomatic in girls. We report three severely affected girls with heterozygous CASK missense mutations (M519T (2), G659D (1)) who exhibit ID, microcephaly, and hindbrain hypoplasia. The mutation M519T results in the replacement of an evolutionarily invariant methionine located in the PDZ signaling domain known to be critical for the CASK-neurexin interaction. CASKM519T is incapable of binding to neurexin, suggesting a critically important role for the CASK-neurexin interaction. The mutation G659D is in the SH3 (Src homology 3) domain of CASK, replacing a semi-conserved glycine with aspartate. We demonstrate that the CASKG659D mutation affects the CASK protein in two independent ways: (1) it increases the protein's propensity to aggregate; and (2) it disrupts the interface between CASK's PDZ (PSD95, Dlg, ZO-1) and SH3 domains, inhibiting the CASK-neurexin interaction despite residing outside of the domain deemed critical for neurexin interaction. Since heterozygosity of other aggregation-inducing mutations (e.g., CASKW919R) does not produce MICPCH, we suggest that the G659D mutation produces microcephaly by disrupting the CASK-neurexin interaction. Our results suggest that disruption of the CASK-neurexin interaction, not the CASK-Tbr-1 interaction, produces microcephaly and cerebellar hypoplasia. These findings underscore the importance of functional validation for variant classification.
Conflict of interest statement
Figures


References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2: 19–25. doi: 10.1016/j.softx.2015.06.001 - DOI
-
- Apic G, Huber W, Teichmann SA (2003) Multi-domain protein families and domain pairs: comparison with known structures and a random model of domain recombination. J Struct Funct Genomics 4: 67–78. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

