A gut feeling: Microbiome-brain-immune interactions modulate social and affective behaviors
- PMID: 29427583
- PMCID: PMC5880698
- DOI: 10.1016/j.yhbeh.2018.02.001
A gut feeling: Microbiome-brain-immune interactions modulate social and affective behaviors
Abstract
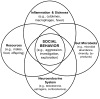
The expression of a wide range of social and affective behaviors, including aggression and investigation, as well as anxiety- and depressive-like behaviors, involves interactions among many different physiological systems, including the neuroendocrine and immune systems. Recent work suggests that the gut microbiome may also play a critical role in modulating behavior and likely functions as an important integrator across physiological systems. Microbes within the gut may communicate with the brain via both neural and humoral pathways, providing numerous avenues of research in the area of the gut-brain axis. We are now just beginning to understand the intricate relationships among the brain, microbiome, and immune system and how they work in concert to influence behavior. The effects of different forms of experience (e.g., changes in diet, immune challenge, and psychological stress) on the brain, gut microbiome, and the immune system have often been studied independently. Though because these systems do not work in isolation, it is essential to shift our focus to the connections among them as we move forward in our investigations of the gut-brain axis, the shaping of behavioral phenotypes, and the possible clinical implications of these interactions. This review summarizes the recent progress the field has made in understanding the important role the gut microbiome plays in the modulation of social and affective behaviors, as well as some of the intricate mechanisms by which the microbiome may be communicating with the brain and immune system.
Keywords: Cytokines; Endocrine system; Gut-brain axis; Immune system; Lipopolysaccharide; Microbiome; Social behavior.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
References
-
- Archie Ea, Theis KR. Animal behaviour meets microbial ecology. Anim Behav. 2011;82:425–436. doi: 10.1016/j.anbehav.2011.05.029. - DOI
-
- Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:1288–1295. doi: 10.1152/ajpgi.00341.2012. - DOI - PubMed
-
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, MacRi J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

