Reproductive and metabolic determinants of granulosa cell dysfunction in normal-weight women with polycystic ovary syndrome
- PMID: 29428312
- PMCID: PMC5812340
- DOI: 10.1016/j.fertnstert.2017.11.017
Reproductive and metabolic determinants of granulosa cell dysfunction in normal-weight women with polycystic ovary syndrome
Abstract
Objective: To determine the degree to which E2 hyperresponsiveness to FSH and antimüllerian hormone (AMH) overproduction in normal-weight women with polycystic ovary syndrome (PCOS) correlate with increased antral follicle number (AFN), hyperandrogenism, and/or metabolic dysfunction.
Design: Prospective cohort study.
Setting: Academic medical center.
Patient(s): Seven normal-weight women with PCOS (1990 National Institutes of Health criteria) ages 20-34 years and 13 age- and body mass index- (BMI-; 18.5-25 kg/m2) matched normoandrogenic ovulatory women were studied.
Intervention(s): All women underwent basal serum hormone and metabolic measurements, FSH stimulation testing with transvaginal ovarian sonography, frequently sampled IV glucose tolerance testing, and whole-body dual-energy x-ray absorptiometry.
Main outcome measure(s): Serum hormone/metabolite levels, 24-hour serum E2 response to 150 IU recombinant human (rh) FSH infusion, AFN, insulin sensitivity, and body mass measurements.
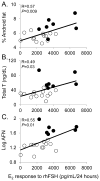
Result(s): Serum E2 responsiveness to rhFSH and AMH levels were greater in women with PCOS than in BMI- and age-matched control women, as were serum androgen levels, AFN, and abdominal fat mass. In all women combined, serum E2 responsiveness to rhFSH was associated with AFN. Serum AMH levels, however, positively correlated with AFN but remained positively correlated with serum LH and free T levels and negatively correlated with total body fat and percent body fat, adjusting for AFN.
Conclusion(s): In normal-weight women with PCOS, serum E2 hyperresponsiveness to rhFSH represents increased AFN, while elevated serum AMH levels reflect opposing effects of stimulatory reproductive (hyperandrogenism and increased AFN) versus inhibitory metabolic (body fat) factors. Given the small number of subjects reported, additional follow-up studies are required to confirm these data.
Keywords: Antimüllerian hormone; PCOS; adiposity; estradiol; hyperandrogenism.
Copyright © 2017 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Dumesic DA, Abbott DH. Accounting for the Follicle Population in the Polycystic Ovary. In: Dunaif A, Chang RJ, Franks S, Legro RS, editors. Polycystic Ovary Syndrome; Current Controversies, from the Ovary to the Pancreas. New York City: Humana Press; 2008. pp. 9–24.
-
- Gougeon A. The early stages of follicular growth. In: Trounson AO, Gosden RG, editors. Biology and Pathology of the Oocyte. Role in Fertility and Reproductive Medicine. Cambridge: Cambridge University Press; 2003. pp. 29–43.
-
- Adashi EY. The ovarian follicular apparatus. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery, and Technology. Philadelphia: Lippincott-Raven; 1996. pp. 18–40.
-
- Faddy MJ, Gosden RG. Modelling the dynamics of ovarian follicle utilization throughout life. In: Trounson AO, Gosden RG, editors. Biology and Pathology of the Oocyte. Role in Fertility and Reproductive Medicine. Cambridge: Cambridge University Press; 2003. pp. 44–52.
-
- Knight PG, Glister C. Local roles of TGF- β superfamily members in the control of ovarian follicle development. Anim Reprod Sci. 2003;78:165–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

