Biotechnology Challenges to In Vitro Maturation of Hepatic Stem Cells
- PMID: 29428334
- PMCID: PMC6237283
- DOI: 10.1053/j.gastro.2018.01.066
Biotechnology Challenges to In Vitro Maturation of Hepatic Stem Cells
Abstract
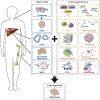
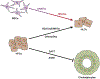
The incidence of liver disease is increasing globally. The only curative therapy for severe end-stage liver disease, liver transplantation, is limited by the shortage of organ donors. In vitro models of liver physiology have been developed and new technologies and approaches are progressing rapidly. Stem cells might be used as a source of liver tissue for development of models, therapies, and tissue-engineering applications. However, we have been unable to generate and maintain stable and mature adult liver cells ex vivo. We review factors that promote hepatocyte differentiation and maturation, including growth factors, transcription factors, microRNAs, small molecules, and the microenvironment. We discuss how the hepatic circulation, microbiome, and nutrition affect liver function, and the criteria for considering cells derived from stem cells to be fully mature hepatocytes. We explain the challenges to cell transplantation and consider future technologies for use in hepatic stem cell maturation, including 3-dimensional biofabrication and genome modification.
Keywords: Culture; Differentiation; Hepatocyte; Liver Development.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
This author discloses the following: A.S.-G. has a provisional patent application that describes hepatic differentiation of human pluripotent stem cells and liver repopulation. A.S.-G. is a co-founder and has a financial interest in Von Baer Wolff, Inc, a company focused on biofabrication of autologous human hepatocytes from stem cells technology. A.S.-G.’s interests are managed by the Conflict of Interest Office at the University of Pittsburgh in accordance with their policies. The remaining authors disclose no conflicts.
Figures

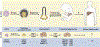

References
-
- Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology 2006;43:345–351. - PubMed
-
- Guidotti JE, Bregerie O, Robert A, et al. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J Biol Chem 2003;278:19095–19101. - PubMed
-
- Gentric G, Desdouets C. Polyploidization in liver tissue. Am J Pathol 2014;184:322–331. - PubMed
-
- Zaret KS. From endoderm to liver bud: paradigms of cell type specification and tissue morphogenesis. Curr Top Dev Biol 2016;117:647–669. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

