The molecular tweezer CLR01 inhibits Ebola and Zika virus infection
- PMID: 29428508
- PMCID: PMC7113745
- DOI: 10.1016/j.antiviral.2018.02.003
The molecular tweezer CLR01 inhibits Ebola and Zika virus infection
Abstract
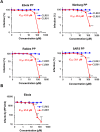
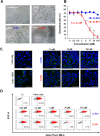
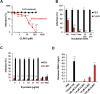
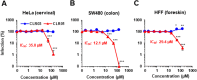
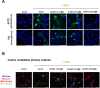
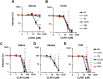
Ebola (EBOV) and Zika viruses (ZIKV) are responsible for recent global health threats. As no preventive vaccines or antiviral drugs against these two re-emerging pathogens are available, we evaluated whether the molecular tweezer CLR01 may inhibit EBOV and ZIKV infection. This small molecule has previously been shown to inactivate HIV-1 and herpes viruses through a selective interaction with lipid-raft-rich regions in the viral envelope, which results in membrane disruption and loss of infectivity. We found that CLR01 indeed blocked infection of EBOV and ZIKV in a dose-dependent manner. The tweezer inhibited infection of epidemic ZIKV strains in cells derived from the anogenital tract and the central nervous system, and remained antivirally active in the presence of semen, saliva, urine and cerebrospinal fluid. Our findings show that CLR01 is a broad-spectrum inhibitor of enveloped viruses with prospects as a preventative microbicide or antiviral agent.
Keywords: Broadly active antiviral agents; Ebola virus; Lipid rafts; Virus inactivation; Zika virus.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

