NDUFB8 Mutations Cause Mitochondrial Complex I Deficiency in Individuals with Leigh-like Encephalomyopathy
- PMID: 29429571
- PMCID: PMC5985356
- DOI: 10.1016/j.ajhg.2018.01.008
NDUFB8 Mutations Cause Mitochondrial Complex I Deficiency in Individuals with Leigh-like Encephalomyopathy
Abstract
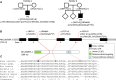
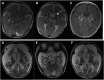
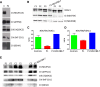
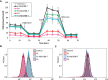
Respiratory chain complex I deficiency is the most frequently identified biochemical defect in childhood mitochondrial diseases. Clinical symptoms range from fatal infantile lactic acidosis to Leigh syndrome and other encephalomyopathies or cardiomyopathies. To date, disease-causing variants in genes coding for 27 complex I subunits, including 7 mitochondrial DNA genes, and in 11 genes encoding complex I assembly factors have been reported. Here, we describe rare biallelic variants in NDUFB8 encoding a complex I accessory subunit revealed by whole-exome sequencing in two individuals from two families. Both presented with a progressive course of disease with encephalo(cardio)myopathic features including muscular hypotonia, cardiac hypertrophy, respiratory failure, failure to thrive, and developmental delay. Blood lactate was elevated. Neuroimaging disclosed progressive changes in the basal ganglia and either brain stem or internal capsule. Biochemical analyses showed an isolated decrease in complex I enzymatic activity in muscle and fibroblasts. Complementation studies by expression of wild-type NDUFB8 in cells from affected individuals restored mitochondrial function, confirming NDUFB8 variants as the cause of complex I deficiency. Hereby we establish NDUFB8 as a relevant gene in childhood-onset mitochondrial disease.
Keywords: Leigh syndrome; NADH dehydrogenase; complex I; lactic acidosis; mitochondria; oxidative phosphorylation; respiratory chain.
Copyright © 2018 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Brandt U. Energy converting NADH:quinone oxidoreductase (complex I) Annu. Rev. Biochem. 2006;75:69–92. - PubMed
-
- Hirst J. Mitochondrial complex I. Annu. Rev. Biochem. 2013;82:551–575. - PubMed
-
- Lazarou M., Thorburn D.R., Ryan M.T., McKenzie M. Assembly of mitochondrial complex I and defects in disease. Biochim. Biophys. Acta. 2009;1793:78–88. - PubMed
-
- Gu J., Wu M., Guo R., Yan K., Lei J., Gao N., Yang M. The architecture of the mammalian respirasome. Nature. 2016;537:639–643. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

