Loss-of-Function Mutations in UNC45A Cause a Syndrome Associating Cholestasis, Diarrhea, Impaired Hearing, and Bone Fragility
- PMID: 29429573
- PMCID: PMC5985364
- DOI: 10.1016/j.ajhg.2018.01.009
Loss-of-Function Mutations in UNC45A Cause a Syndrome Associating Cholestasis, Diarrhea, Impaired Hearing, and Bone Fragility
Abstract
Despite the rapid discovery of genes for rare genetic disorders, we continue to encounter individuals presenting with syndromic manifestations. Here, we have studied four affected people in three families presenting with cholestasis, congenital diarrhea, impaired hearing, and bone fragility. Whole-exome sequencing of all affected individuals and their parents identified biallelic mutations in Unc-45 Myosin Chaperone A (UNC45A) as a likely driver for this disorder. Subsequent in vitro and in vivo functional studies of the candidate gene indicated a loss-of-function paradigm, wherein mutations attenuated or abolished protein activity with concomitant defects in gut development and function.
Keywords: GCUNC-45.
Copyright © 2018 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

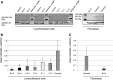

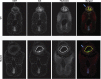
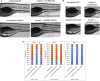
References
-
- Müller T., Hess M.W., Schiefermeier N., Pfaller K., Ebner H.L., Heinz-Erian P., Ponstingl H., Partsch J., Röllinghoff B., Köhler H. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 2008;40:1163–1165. - PubMed
-
- Gonzales E., Taylor S.A., Davit-Spraul A., Thébaut A., Thomassin N., Guettier C., Whitington P.F., Jacquemin E. MYO5B mutations cause cholestasis with normal serum gamma-glutamyl transferase activity in children without microvillous inclusion disease. Hepatology. 2017;65:164–173. - PubMed
-
- Hales C.M., Vaerman J.P., Goldenring J.R. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 2002;277:50415–50421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

