NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control
- PMID: 29429633
- PMCID: PMC5847168
- DOI: 10.1016/j.cell.2018.01.004
NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control
Abstract
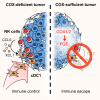
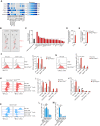
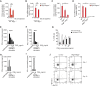
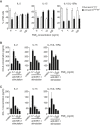
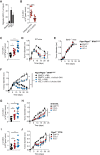
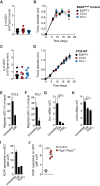
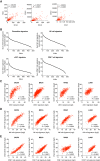
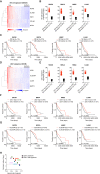
Conventional type 1 dendritic cells (cDC1) are critical for antitumor immunity, and their abundance within tumors is associated with immune-mediated rejection and the success of immunotherapy. Here, we show that cDC1 accumulation in mouse tumors often depends on natural killer (NK) cells that produce the cDC1 chemoattractants CCL5 and XCL1. Similarly, in human cancers, intratumoral CCL5, XCL1, and XCL2 transcripts closely correlate with gene signatures of both NK cells and cDC1 and are associated with increased overall patient survival. Notably, tumor production of prostaglandin E2 (PGE2) leads to evasion of the NK cell-cDC1 axis in part by impairing NK cell viability and chemokine production, as well as by causing downregulation of chemokine receptor expression in cDC1. Our findings reveal a cellular and molecular checkpoint for intratumoral cDC1 recruitment that is targeted by tumor-derived PGE2 for immune evasion and that could be exploited for cancer therapy.
Keywords: cancer immunotherapy; dendritic cells; immune evasion; tumor immune control; tumor microenvironment.
Copyright © 2018 Francis Crick Institute. Published by Elsevier Inc. All rights reserved.
Figures

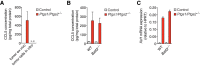

Comment in
-
Tumour immunology: NK cells bring in the troops.Nat Rev Immunol. 2018 Mar;18(3):151. doi: 10.1038/nri.2018.14. Epub 2018 Feb 16. Nat Rev Immunol. 2018. PMID: 29449702 No abstract available.
-
Natural Killer Cells Recruit Dendritic Cells to Promote Antitumor Immunity.Cancer Discov. 2018 Apr;8(4):385. doi: 10.1158/2159-8290.CD-RW2018-030. Epub 2018 Feb 16. Cancer Discov. 2018. PMID: 29453238
-
A team effort: natural killer cells on the first leg of the tumor immunity relay race.J Immunother Cancer. 2018 Jul 9;6(1):67. doi: 10.1186/s40425-018-0380-4. J Immunother Cancer. 2018. PMID: 29986768 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

