Immune or Genetic-Mediated Disruption of CASPR2 Causes Pain Hypersensitivity Due to Enhanced Primary Afferent Excitability
- PMID: 29429934
- PMCID: PMC6011627
- DOI: 10.1016/j.neuron.2018.01.033
Immune or Genetic-Mediated Disruption of CASPR2 Causes Pain Hypersensitivity Due to Enhanced Primary Afferent Excitability
Abstract
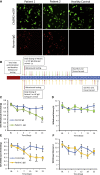
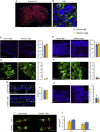
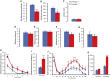
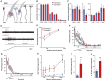
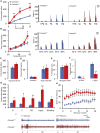
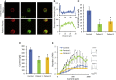
Human autoantibodies to contactin-associated protein-like 2 (CASPR2) are often associated with neuropathic pain, and CASPR2 mutations have been linked to autism spectrum disorders, in which sensory dysfunction is increasingly recognized. Human CASPR2 autoantibodies, when injected into mice, were peripherally restricted and resulted in mechanical pain-related hypersensitivity in the absence of neural injury. We therefore investigated the mechanism by which CASPR2 modulates nociceptive function. Mice lacking CASPR2 (Cntnap2-/-) demonstrated enhanced pain-related hypersensitivity to noxious mechanical stimuli, heat, and algogens. Both primary afferent excitability and subsequent nociceptive transmission within the dorsal horn were increased in Cntnap2-/- mice. Either immune or genetic-mediated ablation of CASPR2 enhanced the excitability of DRG neurons in a cell-autonomous fashion through regulation of Kv1 channel expression at the soma membrane. This is the first example of passive transfer of an autoimmune peripheral neuropathic pain disorder and demonstrates that CASPR2 has a key role in regulating cell-intrinsic dorsal root ganglion (DRG) neuron excitability.
Keywords: CASPR2; CNTNAP2; DRG; Kv1; autism; autoantibody; mechanosensation; pain; sensory neuron; voltage-gated potassium channel.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Autoantibodies Hurt: Transfer of Patient-Derived CASPR2 Antibodies Induces Neuropathic Pain in Mice.Neuron. 2018 Feb 21;97(4):729-731. doi: 10.1016/j.neuron.2018.02.008. Neuron. 2018. PMID: 29470963
References
-
- Arcourt A., Gorham L., Dhandapani R., Prato V., Taberner F.J., Wende H., Gangadharan V., Birchmeier C., Heppenstall P.A., Lechner S.G. Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron. 2017;93:179–193. - PubMed
-
- Carter R.J., Morton J., Dunnett S.B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001;Chapter 8 Unit 8.12. - PubMed
-
- Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

