Low Resting Membrane Potential and Low Inward Rectifier Potassium Currents Are Not Inherent Features of hiPSC-Derived Cardiomyocytes
- PMID: 29429959
- PMCID: PMC5918194
- DOI: 10.1016/j.stemcr.2018.01.012
Low Resting Membrane Potential and Low Inward Rectifier Potassium Currents Are Not Inherent Features of hiPSC-Derived Cardiomyocytes
Abstract
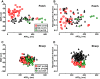
Human induced pluripotent stem cell (hiPSC) cardiomyocytes (CMs) show less negative resting membrane potential (RMP), which is attributed to small inward rectifier currents (IK1). Here, IK1 was measured in hiPSC-CMs (proprietary and commercial cell line) cultured as monolayer (ML) or 3D engineered heart tissue (EHT) and, for direct comparison, in CMs from human right atrial (RA) and left ventricular (LV) tissue. RMP was measured in isolated cells and intact tissues. IK1 density in ML- and EHT-CMs from the proprietary line was similar to LV and RA, respectively. IK1 density in EHT-CMs from the commercial line was 2-fold smaller than in the proprietary line. RMP in EHT of both lines was similar to RA and LV. Repolarization fraction and IK,ACh response discriminated best between RA and LV and indicated predominantly ventricular phenotype in hiPSC-CMs/EHT. The data indicate that IK1 is not necessarily low in hiPSC-CMs, and technical issues may underlie low RMP in hiPSC-CMs.
Keywords: I(K,ACh); I(K1); action potential duration; engineered heart tissue; human atrium; human induced pluripotent stem cell-derived cardiomyocytes; human ventricle; inward rectifier K(+) current; repolarization fraction; resting membrane potential.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




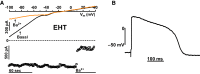

Comment in
-
Leak current, even with gigaohm seals, can cause misinterpretation of stem cell-derived cardiomyocyte action potential recordings.Europace. 2023 Aug 2;25(9):euad243. doi: 10.1093/europace/euad243. Europace. 2023. PMID: 37552789 Free PMC article.
References
-
- Chandler N.J., Greener I.D., Tellez J.O., Inada S., Musa H., Molenaar P., DiFrancesco D., Baruscotti M., Longhi R., Anderson R.H. Molecular architecture of the human sinus node insights into the function of the cardiac pacemaker. Circulation. 2009;119:1562–1575. - PubMed
-
- Chen Z., Xian W., Bellin M., Dorn T., Tian Q., Goedel A., Dreizehnter L., Schneider C.M., Ward-van Oostwaard D., Ng J.K.M. Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in hiPSC-derived cardiomyocytes. Eur. Heart J. 2017;38:292–301. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

