Generation of Functioning Nephrons by Implanting Human Pluripotent Stem Cell-Derived Kidney Progenitors
- PMID: 29429961
- PMCID: PMC5918196
- DOI: 10.1016/j.stemcr.2018.01.008
Generation of Functioning Nephrons by Implanting Human Pluripotent Stem Cell-Derived Kidney Progenitors
Abstract
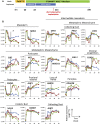
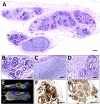
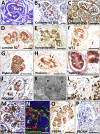
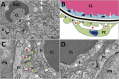
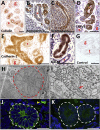
Human pluripotent stem cells (hPSCs) hold great promise for understanding kidney development and disease. We reproducibly differentiated three genetically distinct wild-type hPSC lines to kidney precursors that underwent rudimentary morphogenesis in vitro. They expressed nephron and collecting duct lineage marker genes, several of which are mutated in human kidney disease. Lentiviral-transduced hPSCs expressing reporter genes differentiated similarly to controls in vitro. Kidney progenitors were subcutaneously implanted into immunodeficient mice. By 12 weeks, they formed organ-like masses detectable by bioluminescence imaging. Implants included perfused glomeruli containing human capillaries, podocytes with regions of mature basement membrane, and mesangial cells. After intravenous injection of fluorescent low-molecular-weight dextran, signal was detected in tubules, demonstrating uptake from glomerular filtrate. Thus, we have developed methods to trace hPSC-derived kidney precursors that formed functioning nephrons in vivo. These advances beyond in vitro culture are critical steps toward using hPSCs to model and treat kidney diseases.
Keywords: cell therapy; glomerulus; human embryonic stem cells; kidney; kidney progenitors; lentivirus; metanephric mesenchyme; nephron; ureteric epithelium; vascularization.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Barbaux S., Niaudet P., Gubler M.C., Grünfeld J.P., Jaubert F., Kuttenn F., Fékété C.N., Souleyreau-Therville N., Thibaud E., Fellous M., McElreavey K. Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat. Genet. 1995;17:467–470. - PubMed
-
- Baxter M.A., Camarasa M.V., Bates N., Small F., Murray P., Edgar D., Kimber S.J. Analysis of the distinct functions of growth factors and tissue culture substrates necessary for the long-term self-renewal of human embryonic stem cell lines. Stem Cell Res. 2009;3:28–38. - PubMed
-
- Carpenter M.K., Frey-Vasconcells J., Rao M.S. Developing safe therapies from human pluripotent stem cells. Nat. Biotechnol. 2009;27:606–613. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

