Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system
- PMID: 29430179
- PMCID: PMC5796468
- DOI: 10.2147/IJN.S156930
Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system
Abstract
Background: The use of biopolymers is increasing in drug delivery, thanks to the peculiar properties of these compounds such as their biodegradability, availability, and the possibility of modulating their physico-chemical characteristics. In particular, protein-based systems such as albumin are able to interact with many active compounds, modulating their biopharmaceutical properties. Zein is a protein of 20-40 kDa made up of many hydrophobic amino acids, generally regarded as safe (GRAS) and used as a coating material.
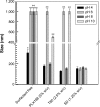
Methods: In this investigation, zein was combined with various surfactants in order to obtain stable nanosystems by means of the nanoprecipitation technique. Specific parameters, eg, temperature, pH value, Turbiscan Stability Index, serum stability, in vitro cytotoxicity and entrapment efficiency of various model compounds were investigated, in order to identify the nanoformulation most useful for a systemic drug delivery application.
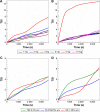
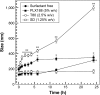
Results: The use of non-ionic and ionic surfactants such as Tween 80, poloxamer 188, and sodium deoxycholate allowed us to obtain nanoparticles characterized by a mean diameter of 100-200 nm when a protein concentration of 2 mg/mL was used. The surface charge was modulated by means of the protein concentration and the nature of the stabilizer. The most suitable nanoparticle formulation to be proposed as a colloidal drug delivery system was obtained using sodium deoxycholate (1.25% w/v) because it was characterized by a narrow size distribution, a good storage stability after freeze-drying and significant feature of retaining lipophilic and hydrophilic compounds.
Conclusion: The sodium deoxycholate-coated zein nanoparticles are stable biocompatible colloidal carriers to be used as useful drug delivery systems.
Keywords: nanoparticles; sodium deoxycholate; stabilizers; zein.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Kim JK, Kim HJ, Chung JY, Lee JH, Young SB, Kim YH. Natural and synthetic biomaterials for controlled drug delivery. Arch Pharm Res. 2014;37(1):60–68. - PubMed
-
- Krats F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132(3):171–183. - PubMed
-
- Reddy N, Yang Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011;29(10):490–498. - PubMed
-
- Irache JM, González-Navarro CJ. Zein nanoparticles as vehicles for oral delivery purposes. Nanomedicine (Lond) 2017;12(11):1209–1211. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

