Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials
- PMID: 29430725
- PMCID: PMC6309613
- DOI: 10.1002/adma.201704847
Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials
Abstract
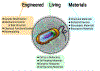
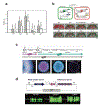
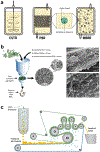
Vast potential exists for the development of novel, engineered platforms that manipulate biology for the production of programmed advanced materials. Such systems would possess the autonomous, adaptive, and self-healing characteristics of living organisms, but would be engineered with the goal of assembling bulk materials with designer physicochemical or mechanical properties, across multiple length scales. Early efforts toward such engineered living materials (ELMs) are reviewed here, with an emphasis on engineered bacterial systems, living composite materials which integrate inorganic components, successful examples of large-scale implementation, and production methods. In addition, a conceptual exploration of the fundamental criteria of ELM technology and its future challenges is presented. Cradled within the rich intersection of synthetic biology and self-assembling materials, the development of ELM technologies allows the power of biology to be leveraged to grow complex structures and objects using a palette of bio-nanomaterials.
Keywords: biomaterials; engineered living materials; self-healing; smart materials; synthetic biology.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




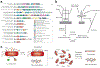









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

