Reassembly of 89 Zr-Labeled Cancer Cell Membranes into Multicompartment Membrane-Derived Liposomes for PET-Trackable Tumor-Targeted Theranostics
- PMID: 29430735
- PMCID: PMC5878718
- DOI: 10.1002/adma.201704934
Reassembly of 89 Zr-Labeled Cancer Cell Membranes into Multicompartment Membrane-Derived Liposomes for PET-Trackable Tumor-Targeted Theranostics
Abstract
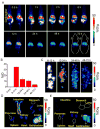
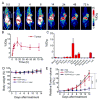
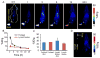
Nanoengineering of cell membranes holds great potential to revolutionize tumor-targeted theranostics, owing to their innate biocompatibility and ability to escape from the immune and reticuloendothelial systems. However, tailoring and integrating cell membranes with drug and imaging agents into one versatile nanoparticle are still challenging. Here, multicompartment membrane-derived liposomes (MCLs) are developed by reassembling cancer cell membranes with Tween-80, and are used to conjugate 89 Zr via deferoxamine chelator and load tetrakis(4-carboxyphenyl) porphyrin for in vivo noninvasive quantitative tracing by positron emission tomography imaging and photodynamic therapy (PDT), respectively. Radiolabeled constructs, 89 Zr-Df-MCLs, demonstrate excellent radiochemical stability in vivo, target 4T1 tumors by the enhanced permeability and retention effect, and are retained long-term for efficient and effective PDT while clearing gradually from the reticuloendothelial system via hepatobiliary excretion. Toxicity evaluation confirms that the MCLs do not impose acute or chronic toxicity in intravenously injected mice. Additionally, 89 Zr-labeled MCLs can execute rapid and highly sensitive lymph node mapping, even for deep-seated sentinel lymph nodes. The as-developed cell membrane reassembling route to MCLs could be extended to other cell types, providing a versatile platform for disease theranostics by facilely and efficiently integrating various multifunctional agents.
Keywords: cancer cell membranes; cancer theranostics; membrane-derived liposomes; positron emission tomography; targeted drug delivery.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA. Nat Nanotechnol. 2013;8:137. - PubMed
-
- Hu Q, Sun W, Lu Y, Bomba HN, Ye Y, Jiang T, Isaacson AJ, Gu Z. Nano Lett. 2016;16:1118. - PubMed
-
- Rao L, Bu LL, Xu JH, Cai B, Yu GT, Yu XL, He ZB, Huang QQ, Li A, Guo SS, Zhang WF, Liu W, Sun ZJ, Wang H, Wang TH, Zhao XZ. Small. 2015;11:6225. - PubMed
-
- Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL. Chem Rev. 2013;113:1904. - PubMed
-
- Chen GY, Roy I, Yang CH, Prasad PN. Chem Rev. 2016;116:2826. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

