Optical Voltage Sensing Using DNA Origami
- PMID: 29430924
- PMCID: PMC5851876
- DOI: 10.1021/acs.nanolett.7b05354
Optical Voltage Sensing Using DNA Origami
Abstract
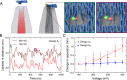
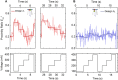
We explore the potential of DNA nanotechnology for developing novel optical voltage sensing nanodevices that convert a local change of electric potential into optical signals. As a proof-of-concept of the sensing mechanism, we assembled voltage responsive DNA origami structures labeled with a single pair of FRET dyes. The DNA structures were reversibly immobilized on a nanocapillary tip and underwent controlled structural changes upon application of an electric field. The applied field was monitored through a change in FRET efficiency. By exchanging the position of a single dye, we could tune the voltage sensitivity of our DNA origami structure, demonstrating the flexibility and versatility of our approach. The experimental studies were complemented by coarse-grained simulations that characterized voltage-dependent elastic deformation of the DNA nanostructures and the associated change in the distance between the FRET pair. Our work opens a novel pathway for determining the mechanical properties of DNA origami structures and highlights potential applications of dynamic DNA nanostructures as voltage sensors.
Keywords: DNA nanotechnology; coarse-grained simulations; nanocapillary; optical voltage measurements; single-molecule FRET.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Hasanzadeh M.; Shadjou N. Electrochemical Nanobiosensing in Whole Blood: Recent Advances. TrAC, Trends Anal. Chem. 2016, 80, 167–176. 10.1016/j.trac.2015.07.018. - DOI
-
- Cetin A. E.; Coskun A. F.; Galarreta B. C.; Huang M.; Herman D.; Ozcan A.; Altug H. Handheld High-Throughput Plasmonic Biosensor Using Computational on-Chip Imaging. Light: Sci. Appl. 2014, 3, e122.10.1038/lsa.2014.3. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

