The metalloprotease ADAM10 (a disintegrin and metalloprotease 10) undergoes rapid, postlysis autocatalytic degradation
- PMID: 29430990
- PMCID: PMC5998973
- DOI: 10.1096/fj.201700823RR
The metalloprotease ADAM10 (a disintegrin and metalloprotease 10) undergoes rapid, postlysis autocatalytic degradation
Abstract
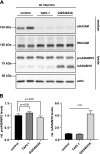
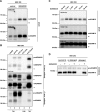
The transmembrane protein, ADAM10 (a disintegrin and metalloprotease 10), has key physiologic functions-for example, during embryonic development and in the brain. During transit through the secretory pathway, immature ADAM10 (proADAM10) is converted into its proteolytically active, mature form (mADAM10). Increasing or decreasing the abundance and/or activity of mADAM10 is considered to be a therapeutic approach for the treatment of such diseases as Alzheimer's disease and cancer. Yet biochemical detection and characterization of mADAM10 has been difficult. In contrast, proADAM10 is readily detected-for example, in immunoblots-which suggests that mADAM10 is only a fraction of total cellular ADAM10. Here, we demonstrate that mADAM10, but not proADAM10, unexpectedly undergoes rapid, time-dependent degradation upon biochemical cell lysis in different cell lines and in primary neurons, which prevents the detection of the majority of mADAM10 in immunoblots. This degradation required the catalytic activity of ADAM10, was efficiently prevented by adding active site inhibitors to the lysis buffer, and did not affect proADAM10, which suggests that ADAM10 degradation occurred in an intramolecular and autoproteolytic manner. Inhibition of postlysis autoproteolysis demonstrated efficient cellular ADAM10 maturation with higher levels of mADAM10 than proADAM10. Moreover, a cycloheximide chase experiment revealed that mADAM10 is a long-lived protein with a half-life of approximately 12 h. In summary, our study demonstrates that mADAM10 autoproteolysis must be blocked to allow for the proper detection of mADAM10, which is essential for the correct interpretation of biochemical and cellular studies of ADAM10.-Brummer, T., Pigoni, M., Rossello, A., Wang, H., Noy, P. J., Tomlinson, M. G., Blobel, C. P., Lichtenthaler, S. F. The metalloprotease ADAM10 (a disintegrin and metalloprotease 10) undergoes rapid, postlysis autocatalytic degradation.
Keywords: ADAM17; Alzheimer’s; GI254023X; NrCAM; tetraspanin15.
Conflict of interest statement
This work was supported by the following funding agencies: Deutsche Forschungsgemeinschaft (FOR2290), the Centers of Excellence in Neurodegeneration, the Helmholtz-Israel Program and the Breuer foundation Alzheimer Award (to S.F.L.), and the U.S. National Institutes of Health, National Institute of General Medical Sciences (Grant R01-GM64750; to C.P.B.). T.B. was supported by a fellowship from the medical faculty of Technische Universität München (Klinikum Rechts der Isar). P.J.N. was funded by the British Heart Foundation Project (Grant PG/13/92/30587; to M.G.T.). The authors thank Linda Troeberg (University of Oxford, Oxford, England) for help with the calculation of
Figures



References
-
- Saftig P., Lichtenthaler S. F. (2015) The alpha secretase ADAM10: a metalloprotease with multiple functions in the brain. Prog. Neurobiol. 135, 1–20 https://doi.org/10.1016/j.pneurobio.2015.10.003 - DOI - PubMed
-
- Wetzel S., Seipold L., Saftig P. (2017) The metalloproteinase ADAM10: a useful therapeutic target? Biochim. Biophys. Acta. 1864, 2071–2081 - PubMed
-
- Jones J. C., Rustagi S., Dempsey P. J. (2016) ADAM proteases and gastrointestinal function. Annu. Rev. Physiol. 78, 243–276 https://doi.org/10.1146/annurev-physiol-021014-071720 - DOI - PMC - PubMed
-
- Weber S., Niessen M. T., Prox J., Lüllmann-Rauch R., Schmitz A., Schwanbeck R., Blobel C. P., Jorissen E., de Strooper B., Niessen C. M., Saftig P. (2011) The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development 138, 495–505 https://doi.org/10.1242/dev.055210 - DOI - PMC - PubMed
-
- Groot A. J., Habets R., Yahyanejad S., Hodin C. M., Reiss K., Saftig P., Theys J., Vooijs M. (2014) Regulated proteolysis of NOTCH2 and NOTCH3 receptors by ADAM10 and presenilins. Mol. Cell. Biol. 34, 2822–2832 https://doi.org/10.1128/MCB.00206-14 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

