Oversized Biodegradable Arterial Grafts Promote Enhanced Neointimal Tissue Formation
- PMID: 29431029
- PMCID: PMC6079650
- DOI: 10.1089/ten.TEA.2017.0483
Oversized Biodegradable Arterial Grafts Promote Enhanced Neointimal Tissue Formation
Abstract
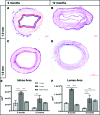
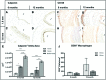
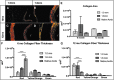
Most tissue-engineered arterial grafts are complicated by aneurysmal dilation secondary to insufficient neotissue formation after scaffold degradation. The optimal graft would form an organized multilayered structure with a robust extracellular matrix that could withstand arterial pressure. The purpose of the current study was to determine how oversizing a biodegradable arterial scaffold affects long-term neotissue formation. Size-matched (1.0 mm, n = 11) and oversized (1.6 mm, n = 9) electrospun polycaprolactone/chitosan scaffolds were implanted as abdominal aortic interposition grafts in Lewis rats. The mean lumen diameter of the 1.6 mm grafts was initially greater compared with the native vessel, but matched the native aorta by 6 months. In contrast, the 1.0 mm grafts experienced stenosis at 6 and 9 months. Total neotissue area and calponin-positive neotissue area were significantly greater in the 1.6 mm grafts by 6 months and similar to the native aorta. Late-term biomechanical testing was dominated by remaining polymer, but graft oversizing did not adversely affect the biomechanics of the adjacent vessel. Oversizing tissue-engineered arterial grafts may represent a strategy to increase the formation of organized neotissue without thrombosis or adverse remodeling of the adjacent native vessel by harnessing a previously undescribed process of adaptive vascular remodeling.
Keywords: chitosan; electrospinning; polycaprolactone; rat model; size mismatch; tissue-engineered arterial graft.
Conflict of interest statement
Dr. C.K.B. is on the Scientific Advisory board of Cook Medical (Bloomington, IN). Dr. C.K.B. receives research support from Gunze, Ltd. (Kyoto Japan) and Cook Regentec (Indianapolis, IN). None of the funding from these grants was used to support this research. Dr. J.J. is a cofounder of Nanofiber Solutions, Inc. (Hilliard, OH). Dr. C.K.B. and Mr. C.B. are cofounders of LYST Therapeutics, LLC (Columbus, OH). Dr. N.H. receives research support from Secant Medical (Telford, PA). Remaining authors have no conflicts of interest to disclose.
Figures




References
-
- World Health Organization. The top 10 causes of death. 2017. http://www.who.int/mediacentre/factsheets/fs310/en/ Accessed October25, 2017
-
- Duval S., Keo H.H., Oldenburg N.C., et al. . The impact of prolonged lower limb ischemia on amputation, mortality, and functional status: the FRIENDS registry. Am Heart J 168, 577, 2014 - PubMed
-
- Yeager R.A., Moneta G.L., Taylor L.M., Hamre D.W., McConnell D.B., and Porter J.M. Surgical management of severe acute lower extremity ischemia. J Vasc Surg 15, 385, 1992 - PubMed
-
- Souza D.S.R., Johansson B., Bojö L., et al. . Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg 132, 373, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

