Hypermetabolic macrophages in rheumatoid arthritis and coronary artery disease due to glycogen synthase kinase 3b inactivation
- PMID: 29431119
- PMCID: PMC6589337
- DOI: 10.1136/annrheumdis-2017-212647
Hypermetabolic macrophages in rheumatoid arthritis and coronary artery disease due to glycogen synthase kinase 3b inactivation
Abstract
Objectives: Accelerated atherosclerotic disease typically complicates rheumatoid arthritis (RA), leading to premature cardiovascular death. Inflammatory macrophages are key effector cells in both rheumatoid synovitis and the plaques of coronary artery disease (CAD). Whether both diseases share macrophage-dependent pathogenic mechanisms is unknown.
Methods: Patients with RA or CAD (at least one myocardial infarction) and healthy age-matched controls were recruited into the study. Peripheral blood CD14+ monocytes were differentiated into macrophages. Metabolic profiles were assessed by Seahorse Analyzer, intracellular ATP concentrations were quantified and mitochondrial protein localisation was determined by confocal image analysis.
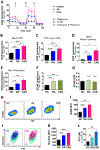
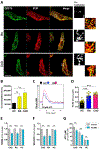
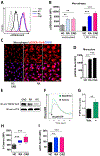
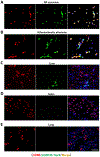
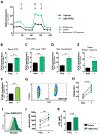
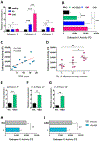
Results: In macrophages from patients with RA or CAD, mitochondria consumed more oxygen, generated more ATP and built tight interorganelle connections with the endoplasmic reticulum, forming mitochondria-associated membranes (MAM). Calcium transfer through MAM sites sustained mitochondrial hyperactivity and was dependent on inactivation of glycogen synthase kinase 3b (GSK3b), a serine/threonine kinase functioning as a metabolic switch. In patient-derived macrophages, inactivated pGSK3b-Ser9 co-precipitated with the mitochondrial fraction. Immunostaining of atherosclerotic plaques and synovial lesions confirmed that most macrophages had inactivated GSK3b. MAM formation and GSK3b inactivation sustained production of the collagenase cathepsin K, a macrophage effector function closely correlated with clinical disease activity in RA and CAD.
Conclusions: Re-organisation of the macrophage metabolism in patients with RA and CAD drives unopposed oxygen consumption and ultimately, excessive production of tissue-destructive enzymes. The underlying molecular defect relates to the deactivation of GSK3b, which controls mitochondrial fuel influx and as such represents a potential therapeutic target for anti-inflammatory therapy.
Keywords: atherosclerosis; cardiovascular disease; inflammation; rheumatoid arthritis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Immunometabolism: Hyperactive macrophages link heart and joint disease.Nat Rev Rheumatol. 2018 Apr;14(4):182. doi: 10.1038/nrrheum.2018.27. Epub 2018 Mar 8. Nat Rev Rheumatol. 2018. PMID: 29515185 No abstract available.
Similar articles
-
Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis.Arthritis Res Ther. 2012 Apr 11;14(2):R74. doi: 10.1186/ar3796. Arthritis Res Ther. 2012. PMID: 22494514 Free PMC article.
-
Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A-dependent pro-inflammatory profile.J Pathol. 2015 Feb;235(3):515-26. doi: 10.1002/path.4466. Epub 2014 Dec 18. J Pathol. 2015. PMID: 25319955
-
CD147 overexpression on synoviocytes in rheumatoid arthritis enhances matrix metalloproteinase production and invasiveness of synoviocytes.Arthritis Res Ther. 2006;8(2):R44. doi: 10.1186/ar1899. Epub 2006 Feb 8. Arthritis Res Ther. 2006. PMID: 16507143 Free PMC article.
-
Metabolic checkpoints in rheumatoid arthritis.Semin Arthritis Rheum. 2025 Feb;70S:152586. doi: 10.1016/j.semarthrit.2024.152586. Epub 2024 Nov 8. Semin Arthritis Rheum. 2025. PMID: 39550308 Review.
-
Synovitis in psoriatic arthritis: immunohistochemistry, comparisons with rheumatoid arthritis, and effects of therapy.Curr Rheumatol Rep. 2011 Aug;13(4):353-9. doi: 10.1007/s11926-011-0181-y. Curr Rheumatol Rep. 2011. PMID: 21503693 Free PMC article. Review.
Cited by
-
Metabolic Control of Autoimmunity and Tissue Inflammation in Rheumatoid Arthritis.Front Immunol. 2021 Apr 2;12:652771. doi: 10.3389/fimmu.2021.652771. eCollection 2021. Front Immunol. 2021. PMID: 33868292 Free PMC article. Review.
-
The transcription factor RFX5 coordinates antigen-presenting function and resistance to nutrient stress in synovial macrophages.Nat Metab. 2022 Jun;4(6):759-774. doi: 10.1038/s42255-022-00585-x. Epub 2022 Jun 23. Nat Metab. 2022. PMID: 35739396 Free PMC article.
-
Dysregulated miR-125a promotes angiogenesis through enhanced glycolysis.EBioMedicine. 2019 Sep;47:402-413. doi: 10.1016/j.ebiom.2019.08.043. Epub 2019 Aug 26. EBioMedicine. 2019. PMID: 31466915 Free PMC article.
-
Reprogramming of synovial macrophage metabolism by synovial fibroblasts under inflammatory conditions.Cell Commun Signal. 2020 Nov 30;18(1):188. doi: 10.1186/s12964-020-00678-8. Cell Commun Signal. 2020. PMID: 33256735 Free PMC article.
-
Acod1-mediated inhibition of aerobic glycolysis suppresses osteoclast differentiation and attenuates bone erosion in arthritis.Ann Rheum Dis. 2024 Nov 14;83(12):1691-1706. doi: 10.1136/ard-2023-224774. Ann Rheum Dis. 2024. PMID: 38964754 Free PMC article.
References
-
- Sack U, Stiehl P, Geiler G. Distribution of macrophages in rheumatoid synovial membrane and its association with basic activity. Rheumatol Int 1994;13(5):181–6. - PubMed
-
- van der Wal AC, Becker AE, van der Loos CM, et al. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89(1):36–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

