Hypermetabolic macrophages in rheumatoid arthritis and coronary artery disease due to glycogen synthase kinase 3b inactivation
- PMID: 29431119
- PMCID: PMC6589337
- DOI: 10.1136/annrheumdis-2017-212647
Hypermetabolic macrophages in rheumatoid arthritis and coronary artery disease due to glycogen synthase kinase 3b inactivation
Abstract
Objectives: Accelerated atherosclerotic disease typically complicates rheumatoid arthritis (RA), leading to premature cardiovascular death. Inflammatory macrophages are key effector cells in both rheumatoid synovitis and the plaques of coronary artery disease (CAD). Whether both diseases share macrophage-dependent pathogenic mechanisms is unknown.
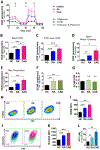
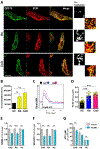
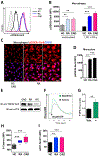
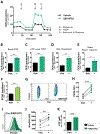
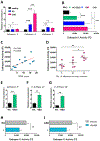
Methods: Patients with RA or CAD (at least one myocardial infarction) and healthy age-matched controls were recruited into the study. Peripheral blood CD14+ monocytes were differentiated into macrophages. Metabolic profiles were assessed by Seahorse Analyzer, intracellular ATP concentrations were quantified and mitochondrial protein localisation was determined by confocal image analysis.
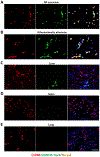
Results: In macrophages from patients with RA or CAD, mitochondria consumed more oxygen, generated more ATP and built tight interorganelle connections with the endoplasmic reticulum, forming mitochondria-associated membranes (MAM). Calcium transfer through MAM sites sustained mitochondrial hyperactivity and was dependent on inactivation of glycogen synthase kinase 3b (GSK3b), a serine/threonine kinase functioning as a metabolic switch. In patient-derived macrophages, inactivated pGSK3b-Ser9 co-precipitated with the mitochondrial fraction. Immunostaining of atherosclerotic plaques and synovial lesions confirmed that most macrophages had inactivated GSK3b. MAM formation and GSK3b inactivation sustained production of the collagenase cathepsin K, a macrophage effector function closely correlated with clinical disease activity in RA and CAD.
Conclusions: Re-organisation of the macrophage metabolism in patients with RA and CAD drives unopposed oxygen consumption and ultimately, excessive production of tissue-destructive enzymes. The underlying molecular defect relates to the deactivation of GSK3b, which controls mitochondrial fuel influx and as such represents a potential therapeutic target for anti-inflammatory therapy.
Keywords: atherosclerosis; cardiovascular disease; inflammation; rheumatoid arthritis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Immunometabolism: Hyperactive macrophages link heart and joint disease.Nat Rev Rheumatol. 2018 Apr;14(4):182. doi: 10.1038/nrrheum.2018.27. Epub 2018 Mar 8. Nat Rev Rheumatol. 2018. PMID: 29515185 No abstract available.
References
-
- Sack U, Stiehl P, Geiler G. Distribution of macrophages in rheumatoid synovial membrane and its association with basic activity. Rheumatol Int 1994;13(5):181–6. - PubMed
-
- van der Wal AC, Becker AE, van der Loos CM, et al. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89(1):36–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

