Does tranexamic acid lead to changes in MRI measures of brain tissue health in patients with spontaneous intracerebral haemorrhage? Protocol for a MRI substudy nested within the double-blind randomised controlled TICH-2 trial
- PMID: 29431141
- PMCID: PMC5879748
- DOI: 10.1136/bmjopen-2017-019930
Does tranexamic acid lead to changes in MRI measures of brain tissue health in patients with spontaneous intracerebral haemorrhage? Protocol for a MRI substudy nested within the double-blind randomised controlled TICH-2 trial
Erratum in
-
Correction: Does tranexamic acid lead to changes in MRI measures of brain tissue health in patients with spontaneous intracerebral haemorrhage? Protocol for a MRI substudy nested within the double-blind randomised controlled TICH-2 trial.BMJ Open. 2018 Apr 20;8(4):e019930corr1. doi: 10.1136/bmjopen-2017-019930corr1. BMJ Open. 2018. PMID: 29678996 Free PMC article. No abstract available.
Abstract
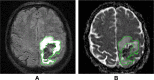
Objectives: To test whether administration of the antifibrinolytic drug tranexamic acid (TXA) in patients with spontaneous intracerebral haemorrhage (SICH) leads to increased prevalence of diffusion-weighted MRI-defined hyperintense ischaemic lesions (primary hypothesis) or reduced perihaematomal oedema volume, perihaematomal diffusion restriction and residual MRI-defined SICH-related tissue damage (secondary hypotheses).
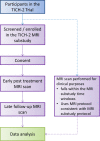
Design: MRI substudy nested within the double-blind randomised controlled Tranexamic Acid for Hyperacute Primary Intracerebral Haemorrhage (TICH)-2 trial (ISRCTN93732214).
Setting: International multicentre hospital-based study.
Participants: Eligible adults consented and randomised in the TICH-2 trial who were also able to undergo MRI scanning. To address the primary hypothesis, a sample size of n=280 will allow detection of a 10% relative increase in prevalence of diffusion-weighted imaging (DWI) hyperintense lesions in the TXA group with 5% significance, 80% power and 5% imaging data rejection.
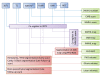
Interventions: TICH-2 MRI substudy participants will undergo MRI scanning using a standardised protocol at day ~5 and day ~90 after randomisation. Clinical assessments, randomisation to TXA or placebo and participant follow-up will be performed as per the TICH-2 trial protocol.
Conclusion: The TICH-2 MRI substudy will test whether TXA increases the incidence of new DWI-defined ischaemic lesions or reduces perihaematomal oedema or final ICH lesion volume in the context of SICH.
Ethics and dissemination: The TICH-2 trial obtained ethical approval from East Midlands - Nottingham 2 Research Ethics Committee (12/EM/0369) and an amendment to allow the TICH-2 MRI sub study was approved in April 2015 (amendment number SA02/15). All findings will be published in peer-reviewed journals. The primary outcome results will also be presented at a relevant scientific meeting.
Trial registration number: ISRCTN93732214; Pre-results.
Keywords: diffusion weighted imaging; hyperacute primary intracerebral haemorrhage; magnetic resonance imaging; perihaematomal oedema; tranexamic acid.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
