Silencing the Snail-Dependent RNA Splice Regulator ESRP1 Drives Malignant Transformation of Human Pulmonary Epithelial Cells
- PMID: 29431637
- PMCID: PMC5899686
- DOI: 10.1158/0008-5472.CAN-17-0315
Silencing the Snail-Dependent RNA Splice Regulator ESRP1 Drives Malignant Transformation of Human Pulmonary Epithelial Cells
Abstract
Epithelial-to-mesenchymal transition (EMT) is organized in cancer cells by a set of key transcription factors, but the significance of this process is still debated, including in non-small cell lung cancer (NSCLC). Here, we report increased expression of the EMT-inducing transcription factor Snail in premalignant pulmonary lesions, relative to histologically normal pulmonary epithelium. In immortalized human pulmonary epithelial cells and isogenic derivatives, we documented Snail-dependent anchorage-independent growth in vitro and primary tumor growth and metastatic behavior in vivo Snail-mediated transformation relied upon silencing of the tumor-suppressive RNA splicing regulatory protein ESRP1. In clinical specimens of NSCLC, ESRP1 loss was documented in Snail-expressing premalignant pulmonary lesions. Mechanistic investigations showed that Snail drives malignant progression in an ALDH+CD44+CD24- pulmonary stem cell subset in which ESRP1 and stemness-repressing microRNAs are inhibited. Collectively, our results show how ESRP1 loss is a critical event in lung carcinogenesis, and they identify new candidate directions for targeted therapy of NSCLC.Significance: This study defines a Snail-ESRP1 cancer axis that is crucial for human lung carcinogenesis, with implications for new intervention strategies and translational opportunities. Cancer Res; 78(8); 1986-99. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures



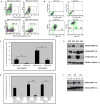



References
-
- Albini A, DeCensi A, Cavalli F, Costa A. Cancer Prevention and Interception: A New Era for Chemopreventive Approaches. Clin Cancer Res. 2016 - PubMed
-
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nature reviews Cancer. 2013;13:97–110. - PubMed
-
- De Craene B, Denecker G, Vermassen P, Taminau J, Mauch C, Derore A, et al. Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 2014;21:310–20. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

