Regulation of Synapse Development by Vgat Deletion from ErbB4-Positive Interneurons
- PMID: 29431653
- PMCID: PMC5858595
- DOI: 10.1523/JNEUROSCI.0669-17.2018
Regulation of Synapse Development by Vgat Deletion from ErbB4-Positive Interneurons
Abstract
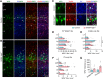
GABA signaling has been implicated in neural development; however, in vivo genetic evidence is missing because mutant mice lacking GABA activity die prematurely. Here, we studied synapse development by ablating vesicular GABA transporter (Vgat) in ErbB4+ interneurons. We show that inhibitory axo-somatic synapses onto pyramidal neurons vary from one cortical layer to another; however, inhibitory synapses on axon initial segments (AISs) were similar across layers. Conversely, parvalbumin-positive (PV+)/ErbB4+ interneurons and PV-only interneurons receive a higher number of inhibitory synapses from PV+ErbB4+ interneurons compared with ErbB4-only interneurons. Vgat deletion from ErbB4+ interneurons reduced axo-somatic or axo-axonic synapses from PV+ErbB4+ interneurons onto excitatory neurons. This effect was associated with corresponding changes in neurotransmission. However, the Vgat mutation seemed to have little effect on inhibitory synapses onto PV+ and/or ErbB4+ interneurons. Interestingly, perineuronal nets, extracellular matrix structures implicated in maturation, survival, protection, and plasticity of PV+ interneurons, were increased in the cortex of ErbB4-Vgat-/- mice. No apparent difference was observed between males and females. These results demonstrate that Vgat of ErbB4+ interneurons is essential for the development of inhibitory synapses onto excitatory neurons and suggest a role of GABA in circuit assembly.SIGNIFICANCE STATEMENT GABA has been implicated in neural development, but in vivo genetic evidence is missing because mutant mice lacking GABA die prematurely. Here, we ablated Vgat in ErbB4+ interneurons in an inducible manner. We provide evidence that the formation of inhibitory and excitatory synapses onto excitatory neurons requires Vgat in interneurons. In particular, inhibitory axo-somatic and axo-axonic synapses are more vulnerable. Our results suggest a role of GABA in circuit assembly.
Keywords: ErbB4; GABA; axo–axonic; axo–somatic; inhibition; parvalbumin.
Copyright © 2018 the authors 0270-6474/18/382533-18$15.00/0.
Figures








References
-
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K (1997) Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A 94:6496–6499. 10.1073/pnas.94.12.6496 - DOI - PMC - PubMed
-
- Bean JC, Lin TW, Sathyamurthy A, Liu F, Yin DM, Xiong WC, Mei L (2014) Genetic labeling reveals novel cellular targets of schizophrenia susceptibility gene: distribution of GABA and non-GABA ErbB4-positive cells in adult mouse brain. J Neurosci 34:13549–13566. 10.1523/JNEUROSCI.2021-14.2014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
