SABRE-Relay: A Versatile Route to Hyperpolarization
- PMID: 29432020
- PMCID: PMC5840861
- DOI: 10.1021/acs.jpclett.7b03026
SABRE-Relay: A Versatile Route to Hyperpolarization
Abstract
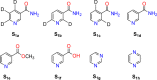
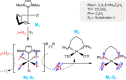
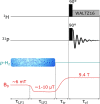
Signal Amplification by Reversible Exchange (SABRE) is used to switch on the latent singlet spin order of para-hydrogen (p-H2) so that it can hyperpolarize a substrate (sub = nicotinamide, nicotinate, niacin, pyrimidine, and pyrazine). The substrate then reacts reversibly with [Pt(OTf)2(bis-diphenylphosphinopropane)] by displacing OTf- to form [Pt(OTf)(sub)(bis-diphenylphosphinopropane)]OTf. The 31P NMR signals of these metal complexes prove to be enhanced when the substrate possesses an accessible singlet state or long-lived Zeeman polarization. In the case of pyrazine, the corresponding 31P signal was 105 ± 8 times larger than expected, which equated to an 8 h reduction in total scan time for an equivalent signal-to-noise ratio under normal acquisition conditions. Hence, p-H2 derived spin order is successfully relayed into a second metal complex via a suitable polarization carrier (sub). When fully developed, we expect this route involving a second catalyst to successfully hyperpolarize many classes of substrates that are not amenable to the original SABRE method.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
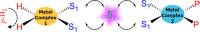
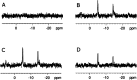
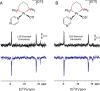
References
-
- Kurhanewicz J.; Vigneron D. B.; Brindle K.; Chekmenev E. Y.; Comment A.; Cunningham C. H.; DeBerardinis R. J.; Green G. G.; Leach M. O.; Rajan S. S.; Rizi R. R.; Ross B. D.; Warren W. S.; Malloy C. R. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia 2011, 13, 81–97. 10.1593/neo.101102. - DOI - PMC - PubMed
-
- Nelson S. J.; Kurhanewicz J.; Vigneron D. B.; Larson P. E. Z.; Harzstark A. L.; Ferrone M.; van Criekinge M.; Chang J. W.; Bok R.; Park I.; Reed G.; Carvajal L.; Small E. J.; Munster P.; Weinberg V. K.; Ardenkjaer-Larsen J. H.; Chen A. P.; Hurd R. E.; Odegardstuen L.-I.; Robb F. J.; Tropp J.; Murray J. A. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized 1-C-13 Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108.10.1126/scitranslmed.3006070. - DOI - PMC - PubMed
-
- Bowers C. R.; Weitekamp D. P. Para-hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alingment. J. Am. Chem. Soc. 1987, 109, 5541–5542. 10.1021/ja00252a049. - DOI
-
- Eisenschmid T. C.; Kirss R. U.; Deutsch P. P.; Hommeltoft S. I.; Eisenberg R.; Bargon J.; Lawler R. G.; Balch A. L. Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc. 1987, 109, 8089–8091. 10.1021/ja00260a026. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

