Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma
- PMID: 29432077
- PMCID: PMC6075856
- DOI: 10.1200/JCO.2017.75.8219
Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma
Abstract
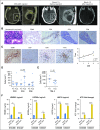
Purpose DNX-2401 (Delta-24-RGD; tasadenoturev) is a tumor-selective, replication-competent oncolytic adenovirus. Preclinical studies demonstrated antiglioma efficacy, but the effects and mechanisms of action have not been evaluated in patients. Methods A phase I, dose-escalation, biologic-end-point clinical trial of DNX-2401 was conducted in 37 patients with recurrent malignant glioma. Patients received a single intratumoral injection of DNX-2401 into biopsy-confirmed recurrent tumor to evaluate safety and response across eight dose levels (group A). To investigate the mechanism of action, a second group of patients (group B) underwent intratumoral injection through a permanently implanted catheter, followed 14 days later by en bloc resection to acquire post-treatment specimens. Results In group A (n = 25), 20% of patients survived > 3 years from treatment, and three patients had a ≥ 95% reduction in the enhancing tumor (12%), with all three of these dramatic responses resulting in > 3 years of progression-free survival from the time of treatment. Analyses of post-treatment surgical specimens (group B, n = 12) showed that DNX-2401 replicates and spreads within the tumor, documenting direct virus-induced oncolysis in patients. In addition to radiographic signs of inflammation, histopathologic examination of immune markers in post-treatment specimens showed tumor infiltration by CD8+ and T-bet+ cells, and transmembrane immunoglobulin mucin-3 downregulation after treatment. Analyses of patient-derived cell lines for damage-associated molecular patterns revealed induction of immunogenic cell death in tumor cells after DNX-2401 administration. Conclusion Treatment with DNX-2401 resulted in dramatic responses with long-term survival in recurrent high-grade gliomas that are probably due to direct oncolytic effects of the virus followed by elicitation of an immune-mediated antiglioma response.
Trial registration: ClinicalTrials.gov NCT00805376.
Figures


Comment in
-
Oncolytic Virotherapy for Malignant Gliomas.J Clin Oncol. 2018 May 10;36(14):1440-1442. doi: 10.1200/JCO.2017.77.3192. Epub 2018 Feb 13. J Clin Oncol. 2018. PMID: 29437534 No abstract available.
-
The Oncolytic Adenovirus DNX-2401 Has Antitumor Activity in Glioblastoma.Cancer Discov. 2018 Apr;8(4):382. doi: 10.1158/2159-8290.CD-RW2018-031. Epub 2018 Feb 23. Cancer Discov. 2018. PMID: 29475886
-
CNS cancer: Oncolytic adenovirus effective in patients with glioblastoma.Nat Rev Clin Oncol. 2018 May;15(5):266. doi: 10.1038/nrclinonc.2018.34. Epub 2018 Feb 27. Nat Rev Clin Oncol. 2018. PMID: 29485135 No abstract available.
References
-
- Wen PY, Kesari S: Malignant gliomas in adults. N Engl J Med 359:492-507, 2008 - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. : Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459-466, 2009 - PubMed
-
- Jiang H, Gomez-Manzano C, Aoki H, et al. : Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: Role of autophagic cell death. J Natl Cancer Inst 99:1410-1414, 2007 - PubMed
-
- Kirn D: Replication-selective oncolytic adenoviruses: Virotherapy aimed at genetic targets in cancer. Oncogene 19:6660-6669, 2000 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

