Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver
- PMID: 29432155
- PMCID: PMC5828596
- DOI: 10.1073/pnas.1715225115
Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver
Abstract
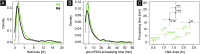
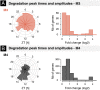
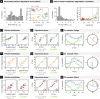
The mammalian circadian clock coordinates physiology with environmental cycles through the regulation of daily oscillations of gene expression. Thousands of transcripts exhibit rhythmic accumulations across mouse tissues, as determined by the balance of their synthesis and degradation. While diurnally rhythmic transcription regulation is well studied and often thought to be the main factor generating rhythmic mRNA accumulation, the extent of rhythmic posttranscriptional regulation is debated, and the kinetic parameters (e.g., half-lives), as well as the underlying regulators (e.g., mRNA-binding proteins) are relatively unexplored. Here, we developed a quantitative model for cyclic accumulations of pre-mRNA and mRNA from total RNA-seq data, and applied it to mouse liver. This allowed us to identify that about 20% of mRNA rhythms were driven by rhythmic mRNA degradation, and another 15% of mRNAs regulated by both rhythmic transcription and mRNA degradation. The method could also estimate mRNA half-lives and processing times in intact mouse liver. We then showed that, depending on mRNA half-life, rhythmic mRNA degradation can either amplify or tune phases of mRNA rhythms. By comparing mRNA rhythms in wild-type and Bmal1-/- animals, we found that the rhythmic degradation of many transcripts did not depend on a functional BMAL1. Interestingly clock-dependent and -independent degradation rhythms peaked at distinct times of day. We further predicted mRNA-binding proteins (mRBPs) that were implicated in the posttranscriptional regulation of mRNAs, either through stabilizing or destabilizing activities. Together, our results demonstrate how posttranscriptional regulation temporally shapes rhythmic mRNA accumulation in mouse liver.
Keywords: RNA binding proteins; circadian clock; mRNA half-lives; posttranscriptional regulation.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015;277:513–527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

