Cysteinyl leukotriene receptor 1 antagonism prevents experimental abdominal aortic aneurysm
- PMID: 29432192
- PMCID: PMC5828611
- DOI: 10.1073/pnas.1717906115
Cysteinyl leukotriene receptor 1 antagonism prevents experimental abdominal aortic aneurysm
Abstract
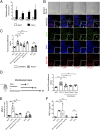
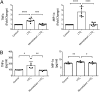
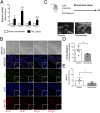
Cysteinyl-leukotrienes (cys-LTs) are 5-lipoxygenase-derived lipid mediators involved in the pathogenesis and progression of inflammatory disorders, in particular asthma. We have previously found evidence linking these mediators to increased levels of proteolytic enzymes in tissue specimens of human abdominal aortic aneurysm (AAA). Here we show that antagonism of the CysLT1 receptor by montelukast, an established antiasthma drug, protects against a strong aorta dilatation (>50% increase = aneurysm) in a mouse model of CaCl2-induced AAA at a dose comparable to human medical practice. Analysis of tissue extracts revealed that montelukast reduces the levels of matrix metalloproteinase-9 (MMP-9) and macrophage inflammatory protein-1α (MIP-1α) in the aortic wall. Furthermore, aneurysm progression was specifically mediated through CysLT1 signaling since a selective CysLT2 antagonist was without effect. A significantly reduced vessel dilatation is also observed when treatment with montelukast is started days after aneurysm induction, suggesting that the drug not only prevents but also stops and possibly reverts an already ongoing degenerative process. Moreover, montelukast reduced the incidence of aortic rupture and attenuated the AAA development in two additional independent models, i.e., angiotensin II- and porcine pancreatic elastase-induced AAA, respectively. Our results indicate that cys-LTs are involved in the pathogenesis of AAA and that antagonism of the CysLT1 receptor is a promising strategy for preventive and therapeutic treatment of this clinically silent and highly lethal disease.
Keywords: abdominal aortic aneurysm; inflammation; leukotriene; montelukast.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Maegdefessel L, Dalman RL, Tsao PS. Pathogenesis of abdominal aortic aneurysms: MicroRNAs, proteases, genetic associations. Annu Rev Med. 2014;65:49–62. - PubMed
-
- Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of AAA progression. Part 1: Extracellular matrix degeneration. Nat Rev Cardiol. 2009;6:464–474. - PubMed
-
- Swedenborg J, Eriksson P. The intraluminal thrombus as a source of proteolytic activity. Ann N Y Acad Sci. 2006;1085:133–138. - PubMed
-
- Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

