GM1 ganglioside-independent intoxication by Cholera toxin
- PMID: 29432456
- PMCID: PMC5825173
- DOI: 10.1371/journal.ppat.1006862
GM1 ganglioside-independent intoxication by Cholera toxin
Abstract
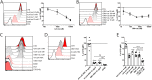
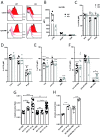
Cholera toxin (CT) enters and intoxicates host cells after binding cell surface receptors via its B subunit (CTB). We have recently shown that in addition to the previously described binding partner ganglioside GM1, CTB binds to fucosylated proteins. Using flow cytometric analysis of primary human jejunal epithelial cells and granulocytes, we now show that CTB binding correlates with expression of the fucosylated Lewis X (LeX) glycan. This binding is competitively blocked by fucosylated oligosaccharides and fucose-binding lectins. CTB binds the LeX glycan in vitro when this moiety is linked to proteins but not to ceramides, and this binding can be blocked by mAb to LeX. Inhibition of glycosphingolipid synthesis or sialylation in GM1-deficient C6 rat glioma cells results in sensitization to CT-mediated intoxication. Finally, CT gavage produces an intact diarrheal response in knockout mice lacking GM1 even after additional reduction of glycosphingolipids. Hence our results show that CT can induce toxicity in the absence of GM1 and support a role for host glycoproteins in CT intoxication. These findings open up new avenues for therapies to block CT action and for design of detoxified enterotoxin-based adjuvants.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. Cholera. Lancet. 2017;390: 1539–1549. doi: 10.1016/S0140-6736(17)30559-7 - DOI - PubMed
-
- Chin C- S, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, et al. The Origin of the Haitian Cholera Outbreak Strain. N Engl J Med. 2011;364: 33–42. doi: 10.1056/NEJMoa1012928 - DOI - PMC - PubMed
-
- Fullner KJ, Lencer WI, Mekalanos JJ. Vibrio cholerae-Induced Cellular Responses of Polarized T84 Intestinal Epithelial Cells Are Dependent on Production of Cholera Toxin and the RTX Toxin. Infection and Immunity. 2001;69: 6310–6317. doi: 10.1128/IAI.69.10.6310-6317.2001 - DOI - PMC - PubMed
-
- Wernick NLB, Chinnapen DJF, Cho JA, Lencer WI. Cholera Toxin: An Intracellular Journey into the Cytosol by Way of the Endoplasmic Reticulum. Toxins. 2010;2: 310–325. doi: 10.3390/toxins2030310 - DOI - PMC - PubMed
-
- Saslowsky DE, Welscher te YM, Chinnapen DJF, Wagner JS, Wan J, Kern E, et al. Ganglioside GM1-mediated transcytosis of cholera toxin bypasses the retrograde pathway and depends on the structure of the ceramide domain. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2013;288: 25804–25809. doi: 10.1074/jbc.M113.474957 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

