Mechanism-based pharmacokinetic (MBPK) models describe the complex plasma kinetics of three antiretrovirals delivered by a long-acting anti-HIV drug combination nanoparticle formulation
- PMID: 29432823
- PMCID: PMC5878144
- DOI: 10.1016/j.jconrel.2018.02.003
Mechanism-based pharmacokinetic (MBPK) models describe the complex plasma kinetics of three antiretrovirals delivered by a long-acting anti-HIV drug combination nanoparticle formulation
Abstract
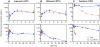
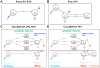
Existing oral antiretroviral (ARV) agents have been shown in human studies to exhibit limited lymph node penetration and lymphatic drug insufficiency. As lymph nodes are a reservoir of HIV, it is critical to deliver and sustain effective levels of ARV combinations in these tissues. To overcome lymph node drug insufficiency of oral combination ARV therapy (cART), we developed and reported a long-acting and lymphocyte-targeting injectable that contains three ARVs-hydrophobic lopinavir (LPV) and ritonavir (RTV), and hydrophilic tenofovir (TFV)-stabilized by lipid excipients in a nanosuspension. A single subcutaneous (SC) injection of this first-generation formulation of drug combination nanoparticles (DcNPs), named TLC-ART101, provided persistent ARV levels in macaque lymph node mononuclear cells (LNMCs) for at least 1 week, and in peripheral blood mononuclear cells (PBMCs) and plasma for at least 2 weeks, demonstrating long-acting pharmacokinetics for all three drugs. In addition, the lymphocyte-targeting properties of this formulation were demonstrated by the consistently higher intracellular drug concentrations in LNMCs and PBMCs versus those in plasma. To provide insights into the complex mechanisms of absorption and disposition of TLC-ART101, we constructed novel mechanism-based pharmacokinetic (MBPK) models. Based upon plasma PK data obtained after single administration of TLC-ART101 (DcNPs) and a solution formulation of free triple-ARVs, the models feature uptake from the SC injection site that respectively routes free and nanoparticle-associated ARVs via the blood vasculature and lymphatics, and their eventual distribution into and clearance from the systemic circulation. The models provided simultaneous description of the complex long-acting plasma and lymphatic PK profiles for all three ARVs in TLC-ART101. The long-acting PK characteristics of the three drugs in TLC-ART101 were likely due to a combination of mechanisms including: (1) DcNPs undergoing preferential lymphatic uptake from the subcutaneous space, (2) retention in nodes during lymphatic first-pass, (3) subsequent slow release of ARVs into blood circulation, and (4) limited extravasation of DcNP-associated ARVs that resulted in longer persistence in the circulation.
Keywords: 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) (PubChem CID: 94190); 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt) (DSPE-mPEG(2000)) (PubChem CID: 406952); Antiretrovirals; HIV drug combination treatment; Long-acting; Lopinavir (PubChem CID: 92727); Lymphatic drug insufficiency; Lymphatic targeted drug delivery; Mechanism-based pharmacokinetic modeling; Ritonavir (PubChem CID: 392622); Tenofovir (PubChem CID: 464205).
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures

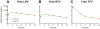


Similar articles
-
Integration of Computational and Experimental Approaches to Elucidate Mechanisms of First-Pass Lymphatic Drug Sequestration and Long-Acting Pharmacokinetics of the Injectable Triple-HIV Drug Combination TLC-ART 101.J Pharm Sci. 2020 May;109(5):1789-1801. doi: 10.1016/j.xphs.2020.01.016. Epub 2020 Jan 29. J Pharm Sci. 2020. PMID: 32006525 Free PMC article.
-
Three HIV Drugs, Atazanavir, Ritonavir, and Tenofovir, Coformulated in Drug-Combination Nanoparticles Exhibit Long-Acting and Lymphocyte-Targeting Properties in Nonhuman Primates.J Pharm Sci. 2018 Dec;107(12):3153-3162. doi: 10.1016/j.xphs.2018.07.032. Epub 2018 Aug 16. J Pharm Sci. 2018. PMID: 30121315 Free PMC article.
-
Long-Acting Profile of 4 Drugs in 1 Anti-HIV Nanosuspension in Nonhuman Primates for 5 Weeks After a Single Subcutaneous Injection.J Pharm Sci. 2018 Jul;107(7):1787-1790. doi: 10.1016/j.xphs.2018.03.005. Epub 2018 Mar 13. J Pharm Sci. 2018. PMID: 29548975 Free PMC article.
-
Recent developments of nanotherapeutics for targeted and long-acting, combination HIV chemotherapy.Eur J Pharm Biopharm. 2019 May;138:75-91. doi: 10.1016/j.ejpb.2018.04.014. Epub 2018 Apr 17. Eur J Pharm Biopharm. 2019. PMID: 29678735 Free PMC article. Review.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
Cited by
-
Dendritic Cell-Hitchhiking In Vivo for Vaccine Delivery to Lymph Nodes.Adv Sci (Weinh). 2024 Sep;11(33):e2402199. doi: 10.1002/advs.202402199. Epub 2024 Jul 4. Adv Sci (Weinh). 2024. PMID: 38962939 Free PMC article.
-
Novel Long-Acting Drug Combination Nanoparticles Composed of Gemcitabine and Paclitaxel Enhance Localization of Both Drugs in Metastatic Breast Cancer Nodules.Pharm Res. 2020 Sep 23;37(10):197. doi: 10.1007/s11095-020-02888-8. Pharm Res. 2020. PMID: 32968837 Free PMC article.
-
Mass Spectrometry Imaging Demonstrates the Regional Brain Distribution Patterns of Three First-Line Antiretroviral Drugs.ACS Omega. 2019 Dec 5;4(25):21169-21177. doi: 10.1021/acsomega.9b02582. eCollection 2019 Dec 17. ACS Omega. 2019. PMID: 31867510 Free PMC article.
-
Physiologically based Pharmacokinetic Model Validated to Enable Predictions Of Multiple Drugs in a Long-acting Drug-combination Nano-Particles (DcNP): Confirmation with 3 HIV Drugs, Lopinavir, Ritonavir, and Tenofovir in DcNP Products.J Pharm Sci. 2024 Jun;113(6):1653-1663. doi: 10.1016/j.xphs.2024.02.018. Epub 2024 Feb 20. J Pharm Sci. 2024. PMID: 38382809 Free PMC article.
-
A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives.Acta Pharm Sin B. 2021 Aug;11(8):2396-2415. doi: 10.1016/j.apsb.2021.05.002. Epub 2021 Jun 17. Acta Pharm Sin B. 2021. PMID: 34522592 Free PMC article. Review.
References
-
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, Lutz T, Angel JB, Richmond GJ, Clotet B, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. - PubMed
-
- Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, Piscitelli S. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67(5):481–486. - PubMed
-
- McGowan I, Dezzutti CS, Siegel A, Engstrom J, Nikiforov A, Duffill K, Shetler C, Richardson-Harman N, Abebe K, Back D, et al. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV. 2016;3(12):e569–e578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

