GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence
- PMID: 29432858
- PMCID: PMC6692166
- DOI: 10.1016/j.jclinepi.2018.01.012
GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence
Abstract
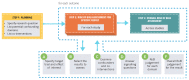
Objective: To provide guidance on how systematic review authors, guideline developers, and health technology assessment practitioners should approach the use of the risk of bias in nonrandomized studies of interventions (ROBINS-I) tool as a part of GRADE's certainty rating process.
Study design and setting: The study design and setting comprised iterative discussions, testing in systematic reviews, and presentation at GRADE working group meetings with feedback from the GRADE working group.
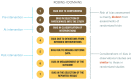
Results: We describe where to start the initial assessment of a body of evidence with the use of ROBINS-I and where one would anticipate the final rating would end up. The GRADE accounted for issues that mitigate concerns about confounding and selection bias by introducing the upgrading domains: large effects, dose-effect relations, and when plausible residual confounders or other biases increase certainty. They will need to be considered in an assessment of a body of evidence when using ROBINS-I.
Conclusions: The use of ROBINS-I in GRADE assessments may allow for a better comparison of evidence from randomized controlled trials (RCTs) and nonrandomized studies (NRSs) because they are placed on a common metric for risk of bias. Challenges remain, including appropriate presentation of evidence from RCTs and NRSs for decision-making and how to optimally integrate RCTs and NRSs in an evidence assessment.
Keywords: Certainty of the evidence; GRADE; Nonrandomized studies; Quality of evidence; ROBINS; Risk of bias.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
HJS has no direct financial conflict of interest and other authors have not declared financial conflicts of interest. Part of the work has been presented scientific conferences and at GRADE Working Group meetings. This article has been officially endorsed by the GRADE Working Group.
Figures



References
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. - PubMed
-
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knotterus A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2010 - PubMed
-
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. - PubMed
-
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP J Club. 2008;149(6):2. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

