Nutraceuticals: opening the debate for a regulatory framework
- PMID: 29433155
- PMCID: PMC5867125
- DOI: 10.1111/bcp.13496
Nutraceuticals: opening the debate for a regulatory framework
Abstract
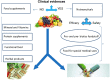
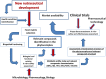
Currently, nutraceuticals do not have a specific definition distinct from those of other food-derived categories, such as food supplements, herbal products, pre- and probiotics, functional foods, and fortified foods. Many studies have led to an understanding of the potential mechanisms of action of pharmaceutically active components contained in food that may improve health and reduce the risk of pathological conditions while enhancing overall well-being. Nevertheless, there is a lack of clear information and, often, the claimed health benefits may not be properly substantiated by safety and efficacy information or in vitro and in vivo data, which can induce false expectations and miss the target for a product to be effective, as claimed. An officially shared and accepted definition of nutraceuticals is still missing, as nutraceuticals are mostly referred to as pharma-foods, a powerful toolbox to be used beyond the diet but before the drugs to prevent and treat pathological conditions, such as in subjects who may not yet be eligible for conventional pharmaceutical therapy. Hence, it is of utmost importance to have a proper and unequivocal definition of nutraceuticals and shared regulations. It also seems wise to assess the safety, mechanism of action and efficacy of nutraceuticals with clinical data. A growing demand exists for nutraceuticals, which seem to reside in the grey area between pharmaceuticals and food. Nonetheless, given specific legislation from different countries, nutraceuticals are experiencing challenges with safety and health claim substantiation.
Keywords: claims; health; labels; nutraceuticals; regulation; regulatory.
© 2018 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures
References
-
- Borchers AT, Keen CL, Gerswin ME. The basis of structure/function claims of nutraceuticals. Clin Rev Allergy Immunol 2016; 51: 370–382. - PubMed
-
- DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol 1995; 6: 59–61.
-
- El Sohaimy SA. Functional foods and nutraceuticals‐modern approach to food science. World Appl Sci J 2012; 20: 691–708.
-
- Santini A, Novellino E. Nutraceuticals: beyond the diet before the drugs. Curr Bioact Compd 2014; 10: 1–12.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical