A randomized controlled trial of an intervention delivered by mobile phone app instant messaging to increase the acceptability of effective contraception among young people in Tajikistan
- PMID: 29433506
- PMCID: PMC5809875
- DOI: 10.1186/s12978-018-0473-z
A randomized controlled trial of an intervention delivered by mobile phone app instant messaging to increase the acceptability of effective contraception among young people in Tajikistan
Erratum in
-
Correction to: A randomized controlled trial of an intervention delivered by mobile phone app instant messaging to increase the acceptability of effective contraception among young people in Tajikistan.Reprod Health. 2018 Mar 26;15(1):52. doi: 10.1186/s12978-018-0496-5. Reprod Health. 2018. PMID: 29580246 Free PMC article.
Abstract
Background: Unintended pregnancy is associated with poorer health outcomes for women and their families. In Tajikistan, around 26% of married 15-24 year old women have an unmet need for contraception. There is some evidence that interventions delivered by mobile phone can affect contraceptive-related behaviour and knowledge. We developed an intervention delivered by mobile phone app instant messaging to improve acceptability of effective contraceptive methods among young people in Tajikistan.
Methods: This was a randomized controlled trial among Tajik people aged 16-24. Participants allocated to the intervention arm had access to an app plus intervention messages. Participants allocated to the control arm had access to the app plus control messages. The primary outcome was acceptability of at least one method of effective contraception at 4 months. Secondary outcomes were use of effective contraception at 4 months and during the study, acceptability of individual methods, service uptake, unintended pregnancy and induced abortion. Process outcomes were knowledge, perceived norms, personal agency and intention. Outcomes were analysed using logistic and linear regression. We conducted a pre-specified subgroup analysis and a post-hoc analysis of change in acceptability from baseline to follow-up.
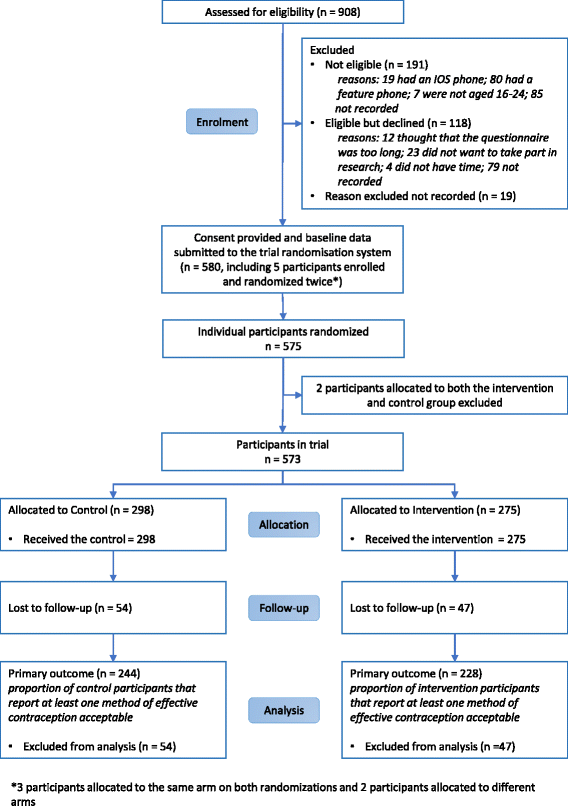
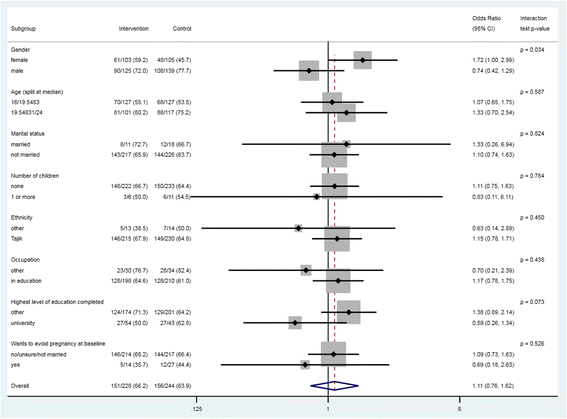
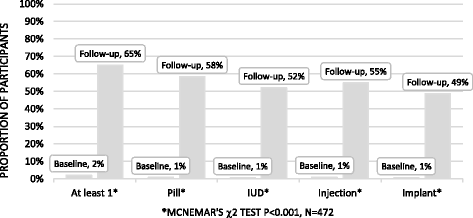
Results: Five hundred and seventy-three participants were enrolled. Intervention content was included on the app, causing contamination. Four hundred and seventy-two (82%) completed follow-up for the primary outcome. There was no evidence of a difference in acceptability of effective contraception between the groups (66% in the intervention arm vs 64% in the control arm, adjusted OR 1.21, 95% CI .80-1.83, p = 0.36). There were no differences in the secondary or process outcomes between groups. There was some evidence that the effect of the intervention was greater among women compared to men (interaction test p = 0.03). There was an increase in acceptability of effective contraception from baseline to follow-up (2% to 65%, p < 0.001).
Conclusions: The whole intervention delivered by instant messaging provided no additional benefit over a portion of the intervention delivered by app pages. The important increase in contraceptive acceptability from baseline to follow-up suggests that the intervention content included on the app may influence attitudes. Further research is needed to establish the effect of the intervention on attitudes towards and use of effective contraception among married/sexually active young people.
Trial registration: Clinicaltrial.gov NCT02905513 . Date of registration: 14 September 2016.
Keywords: Contraception; Randomized controlled trial; Reproductive health; Smart phone; Tajikistan; Young adults.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was granted from the London School of Hygiene and Tropical Medicine Observational Research Ethics Committee on 27 April 2015 (reference number 9148), the Tajik National Scientific and Research Centre on Pediatrics and Child Surgery on 8 June 2015.
All participants provided informed consent before providing baseline data and randomization.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Logan C, Holcombe E, Manlove J, Ryan S. The consequences of unintended childbearing: a white paper. Washington, DC: Child Trends; 2007.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical