Effect of Mobile-health on maternal health care service utilization in Eastern Ethiopia: study protocol for a randomized controlled trial
- PMID: 29433537
- PMCID: PMC5809837
- DOI: 10.1186/s13063-018-2446-5
Effect of Mobile-health on maternal health care service utilization in Eastern Ethiopia: study protocol for a randomized controlled trial
Abstract
Background: Globally, the rapid development of mobile technology has created new ways of addressing public health challenges and shifted the paradigm of health care access and delivery. The primary aim of this study is to examine the effectiveness of Mobile-health on maternal health care service utilization in Eastern Ethiopia.
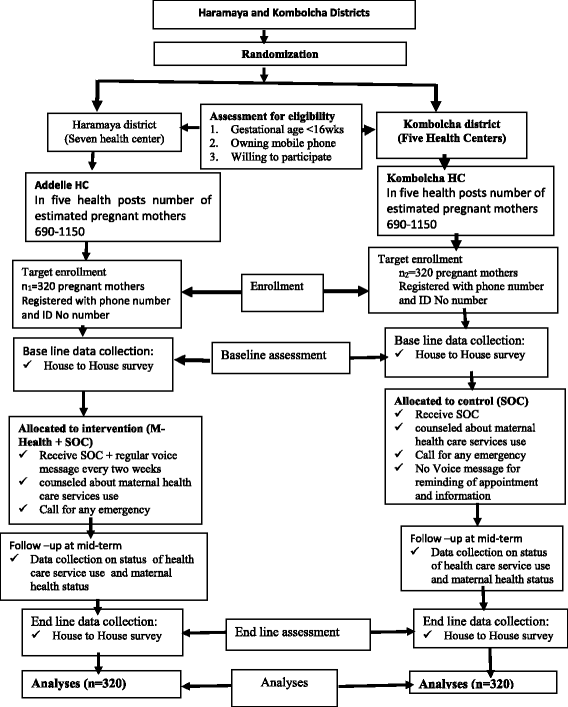
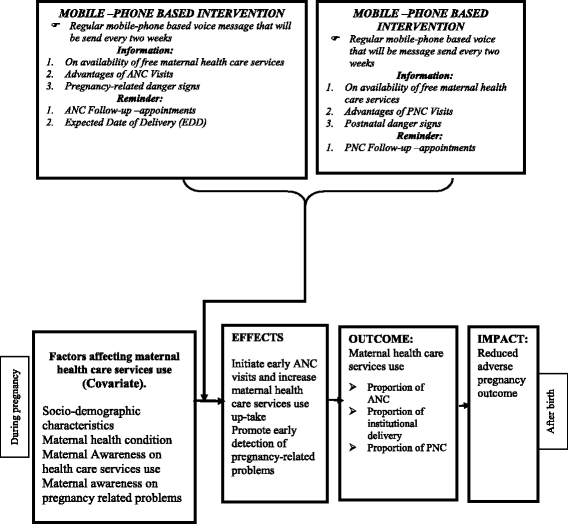
Methods/design: Through, a cluster-randomized controlled trial, 640 participants will be selected based on their districts and respective health centers as the unit of randomization. All pregnant mothers who fulfill the inclusion criteria will be allocated to a mobile-phone-based intervention and existing standard of care or control with a 1:1 allocation ratio. The intervention consists of a series of 24 voice messages which will be sent every 2 weeks from the date of enrollment until the close-out time. The control group will receive existing standard of care without voice messages. Data related to outcome variables will be assessed at three phases of the data collection periods. The primary outcome measures will be the proportion of antenatal care visits and institutional delivery, whereas the secondary outcome measures will consist of the proportion of postnatal care visits and pregnancy outcomes. Risk ratios will be used to a measure the effect of intervention on the outcomes which will be estimated with 95% confidence interval and all the analyses will be done with consideration of clustering effect.
Discussions: This study should generate evidence on the effectiveness of mobile-phone-based voice messages for the early initiation of maternal health care service use and its uptake. It has been carefully designed with the assumption of obtaining higher levels of maternal health care service use among the treatment group as compared to the control.
Trial registration: Pan African Clinical Trial Registry, www.panctr.org , ID: PACTR201704002216259 . Registered on 28 April 2017.
Keywords: Eastern Ethiopia; Maternal health; Mobile-health; Randomized controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
The trials will be conducted in line with the principles of Good Clinical Practice [34]. Ethical approval for the study was granted from Haramaya University, IHRERC, College of Health and Medical Sciences, Human Research Ethics Review Committee (Ref. No. IHRERC/127/2017), 8 May 2017. All the participants will provide written informed consent. This trial study has been registered at the Pan African Clinical Trial Registry,
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Union IT. ICT facts and figures of the world. Geneva. 2010.
-
- INSEAD, World Economic Forum. The Global Information Technology Report. In: Dutta S, Lanvin B, Bilbao-Osorio B, editors. Growth and jobs in a hyperconnected world. Geneva; 2013.
-
- Mechael P, Batavia H, Kaonga N, Searle S, Kwan A, Goldberger A. Barriers and gaps affecting mhealth in low and middle income countries. In: Policy white paper. New York: Earth Institute; 2010.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

