Murine hematopoietic stem cell reconstitution potential is maintained by osteopontin during aging
- PMID: 29434282
- PMCID: PMC5809550
- DOI: 10.1038/s41598-018-21324-x
Murine hematopoietic stem cell reconstitution potential is maintained by osteopontin during aging
Abstract
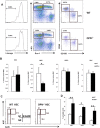
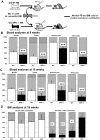
In adult mammals, hematopoietic stem cells (HSCs) reside in the bone marrow and are in part regulated by the bone marrow microenvironment, called the stem cell niche. We have previously identified the bone marrow morphogen osteopontin (OPN), which is abundantly present in the bone marrow extracellular matrix, as a negative regulator of the size of the HSC pool under physiological conditions. Here, we study the impact of OPN on HSC function during aging using an OPN-knockout mouse model. We show that during aging OPN deficiency is associated with an increase in lymphocytes and a decline in erythrocytes in peripheral blood. In a bone marrow transplantation setting, aged OPN-deficient stem cells show reduced reconstitution ability likely due to insufficient differentiation of HSCs into more mature cells. In serial bone marrow transplantation, aged OPN-/- bone marrow cells fail to adequately reconstitute red blood cells and platelets, resulting in severe anemia and thrombocytopenia as well as premature deaths of recipient mice. Thus, OPN has different effects on HSCs in aged and young animals and is particularly important to maintain stem cell function in aging mice.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Beerman I, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

