Acute Hepatocellular Jaundice After Dofetilide Initiation: A Case Report
- PMID: 29434388
- PMCID: PMC5805014
- DOI: 10.1177/0018578717738079
Acute Hepatocellular Jaundice After Dofetilide Initiation: A Case Report
Abstract
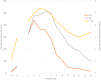
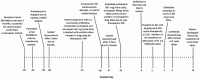
Dofetilide's hepatotoxicity is not well described. In this case report, we describe acute hepatocellular jaundice related to dofetilide use in a 33-year-old male being treated for atrial fibrillation. Both viral and ischemic causes of hepatocellular damage were ruled out as unlikely in this case. This case report outlines a rare yet probable report of idiosyncratic dofetilide-induced liver injury.
Keywords: adverse drug reactions; adverse drug reactions reporting/monitoring; cardiac agents; cardiovascular; gastrointestinal disorders.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Mounsey JP, DiMarco JP. Dofetilide. Circulation. 2000;102(21):2665-2670. - PubMed
-
- Pfizer Inc. Tikosyn (dofetilide capsule). In: DailyMed. New York, NY: US National Library of Medicine; https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=02438044-d6a3-4.... Accessed October 10, 2017.
-
- Kootte AM, Janssens AR, Ouwehand DK, van Leeuwen AM. [Chronic hepatitis ascribed to the use of sotalol]. Ned Tijdschr Geneeskd. 2001;145(48):2340-2343. - PubMed
-
- Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966; quiz 967. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

