Identification of a Novel CD8 T Cell Epitope Derived from Plasmodium berghei Protective Liver-Stage Antigen
- PMID: 29434602
- PMCID: PMC5796907
- DOI: 10.3389/fimmu.2018.00091
Identification of a Novel CD8 T Cell Epitope Derived from Plasmodium berghei Protective Liver-Stage Antigen
Abstract
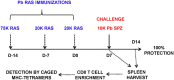
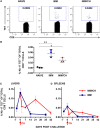
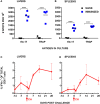
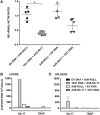
We recently identified novel Plasmodium berghei (Pb) liver stage (LS) genes that as DNA vaccines significantly reduce Pb LS parasite burden (LPB) in C57Bl/6 (B6) mice through a mechanism mediated, in part, by CD8 T cells. In this study, we sought to determine fine antigen (Ag) specificities of CD8 T cells that target LS malaria parasites. Guided by algorithms for predicting MHC class I-restricted epitopes, we ranked sequences of 32 Pb LS Ags and selected ~400 peptides restricted by mouse H-2Kb and H-2Db alleles for analysis in the high-throughput method of caged MHC class I-tetramer technology. We identified a 9-mer H-2Kb restricted CD8 T cell epitope, Kb-17, which specifically recognized and activated CD8 T cell responses in B6 mice immunized with Pb radiation-attenuated sporozoites (RAS) and challenged with infectious sporozoites (spz). The Kb-17 peptide is derived from the recently described novel protective Pb LS Ag, PBANKA_1031000 (MIF4G-like protein). Notably, immunization with the Kb-17 epitope delivered in the form of a minigene in the adenovirus serotype 5 vector reduced LPB in mice infected with spz. On the basis of our results, Kb-17 peptide was available for CD8 T cell activation and recall following immunization with Pb RAS and challenge with infectious spz. The identification of a novel MHC class I-restricted epitope from the protective Pb LS Ag, MIF4G-like protein, is crucial for advancing our understanding of immune responses to Plasmodium and by extension, toward vaccine development against malaria.
Keywords: CD8 T cells; Plasmodium; caged MHC-tetramers; epitope prediction; minigene.
Figures
References
-
- WHO. World Malaria Report 2016. World Health Organization; (2016).
-
- Reece WH, Pinder M, Gothard PK, Milligan P, Bojang K, Doherty T, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med (2004) 10(4):406–10.10.1038/nm1009 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

