The Role of the Endothelium during Antibody-Mediated Rejection: From Victim to Accomplice
- PMID: 29434607
- PMCID: PMC5796908
- DOI: 10.3389/fimmu.2018.00106
The Role of the Endothelium during Antibody-Mediated Rejection: From Victim to Accomplice
Abstract
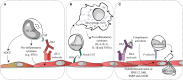
Antibody-mediated rejection (AMR) of solid organ transplants is characterized by the activation and injury of the allograft endothelium. Histological and transcriptomic studies have associated microvascular inflammation and endothelial lesions with the severity of rejection and poor graft outcomes. The allograft endothelium forms the physical barrier between the donor organ and the recipient; this position directly exposes the endothelium to alloimmune responses. However, endothelial cells are not just victims and can actively participate in the pathogenesis of rejection. In healthy tissues, the endothelium plays a major role in vascular and immune homeostasis. Organ transplantation, however, subjects the endothelium to an environment of inflammation, alloreactive lymphocytes, donor-specific antibodies, and potentially complement activation. As a result, endothelial cells become activated and have modified interactions with the cellular effectors of allograft damage: lymphocytes, natural killer, and myeloid cells. Activated endothelial cells participate in leukocyte adhesion and recruitment, lymphocyte activation and differentiation, as well as the secretion of cytokines and chemokines. Ultimately, highly activated endothelial cells promote pro-inflammatory alloresponses and become accomplices to AMR.
Keywords: antibody-mediated rejection; donor-specific antibodies; endothelial cells; inflammation; transplantation immunology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

