Function of Axl receptor tyrosine kinase in non-small cell lung cancer
- PMID: 29434997
- PMCID: PMC5778882
- DOI: 10.3892/ol.2017.7694
Function of Axl receptor tyrosine kinase in non-small cell lung cancer
Abstract
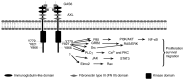
Axl receptor tyrosine kinase (hereafter Axl) is a member of the tyrosine-protein kinase receptor Tyro3, Axl and proto-oncogene tyrosine-protein kinase Mer family of receptor tyrosine kinases, possessing multiple different functions in normal cells. Axl is overexpressed and activated in numerous different human cancer types, triggering several signaling pathways and enhancing tumor progression. The present review assesses previous studies on the function of Axl in non-small cell lung cancer (NSCLC). Axl is overexpressed in the tumor tissues of a number of patients with NSCLC and is associated with poorer clinical outcomes; it promotes NSCLC tumor growth, invasion/metastasis, drug resistance and the epithelial-mesenchymal transition, thus providing a survival advantage to tumor cells. Therefore, Axl may be a promising target in NSCLC treatment.
Keywords: Axl receptor tyrosine kinase; drug resistance; epithelial-mesenchymal transition; invasion; metastasis; non-small cell lung cancer.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
