Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2
- PMID: 29435364
- PMCID: PMC5761290
- DOI: 10.1136/rmdopen-2017-000592
Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2
Abstract
Background: Secukinumab treatment has previously been shown to significantly improve the signs and symptoms of active ankylosing spondylitis (AS), with responses sustained through 2 years. Here, we report the long-term (3 years) efficacy and safety of secukinumab in the MEASURE 2 study.
Methods: MEASURE 2 (NCT01649375) is a 5-year phase III, randomised, double-blind, double-dummy, parallel-group, placebo-controlled study to evaluate the efficacy, safety and tolerability of subcutaneous loading and maintenance dosing of secukinumab in adult subjects with active AS. Subjects were randomised to receive subcutaneous secukinumab 150 mg, 75 mg or placebo at baseline, weeks 1, 2 and 3 and every 4 weeks from week 4. At week 16, placebo-treated subjects were rerandomised to receive secukinumab 150/75 mg.
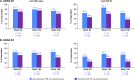
Results: Retention rates were high during weeks 16-156 and were 86% and 76% for secukinumab 150 and 75 mg, respectively. Secukinumab 150 mg provided sustained improvements in the Assessment of Spondyloarthritis International Society ASAS 20/40 response rates at week 156 (70.1%/60.9%) compared with week 52 (74.2%/57.0%); however, there was a slight decrease for secukinumab 75 mg (54.3%/37.0% vs 62.5%/43.2%, respectively). Sustained improvements were observed in all other end points, including Bath Ankylosing Spondylitis Disease Activity Index, AS Disease Activity Score with C reactive protein inactive disease, ASAS 5/6, Short Form-36 Physical Component Summary and ASAS partial remission. Clinical benefits were observed regardless of prior exposure to anti-tumour necrosis factor agents. The safety profile remained favourable and was consistent with previous reports.
Conclusions: This study showed sustained improvement through 3 years in signs, symptoms and physical function in subjects with AS. Retention rates were high and secukinumab was well tolerated, with a favourable safety profile.
Keywords: ankylosing spondylitis; cytokines; dmards (biologic); spondyloarthritis.
Conflict of interest statement
Competing interests: HM-O: grant/research support from Janssen and Celgene; speaker fees from Janssen, Pfizer, AbbVie, Celgene, Novartis and UCB. JS: grant/research support from AbbVie, Pfizer and Merck, consultant for AbbVie, Pfizer, Merck, UCB and Novartis; speaker support from: AbbVie, Pfizer, Merck and UCB. AK: grant/research support from Altoona Center for Clinical Research, PC; consultant fess from Vertex, AbbVie, Amgen, Celgene, Horizon, Genetech, Janssen, Merck, Novartis, Pfizer, UCB, Genzyme, Sanofi, Regeneron, SUN Pharma Advanced Research, Boehringer Ingelheim. RB: grants/research support from AbbVie, MSD and Roche; consultant fees/speaker support from AbbVie, Pfizer, Roche, Bristol-Myers, Janssen, Lilly and MSD. MC: consultant fees/speaker support from AbbVie, Amgen, BMS, Celgene, Janssen, Lilly, Merck, Novartis, Paladin, Pfizer, Roche, Sanofi, UCB. EMD: employee of Novartis. SR: employee of, and owns stock, in Novartis. HR: employee of, and owns stock, in Novartis.
Figures

References
-
- Ward MM, Deodhar A, Akl EA, et al. . American college of rheumatology/spondylitis association of America/spondyloarthritis research and treatment network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2016;68:282–98. 10.1002/art.39298 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
