Wearable Chemosensors: A Review of Recent Progress
- PMID: 29435397
- PMCID: PMC5792729
- DOI: 10.1002/open.201700159
Wearable Chemosensors: A Review of Recent Progress
Abstract
In recent years, there has been growing demand for wearable chemosensors for their important potential applications in mobile and electronic healthcare, patient self-assessment, human motion monitoring, and so on. Innovations in wearable chemosensors are revolutionizing the modern lifestyle, especially the involvement of both doctors and patients in the modern healthcare system. The facile interaction of wearable chemosensors with the human body makes them favorable and convenient tools for the detection and long-term monitoring of the chemical, biological, and physical status of the human body at a low cost with high performance. In this Minireview, we give a brief overview of the recent advances and developments in the field of wearable chemosensors, summarize the basic types of wearable chemosensors, and discuss their main functions and fabrication methods. At the end of this paper, the future development direction of wearable chemosensors is prospected. With continued interest and attention to this field, new exciting progress is expected in the development of innovative wearable chemosensors.
Keywords: analytical methods; biosensors; electronic healthcare; sensors; wearable chemosensors.
Figures

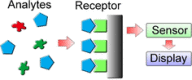


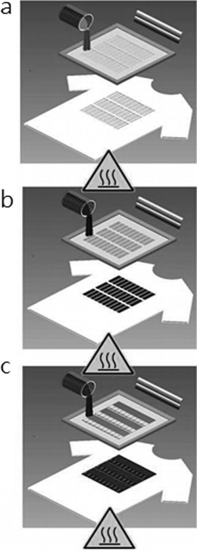


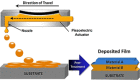
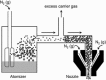

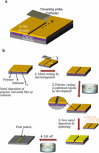
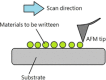


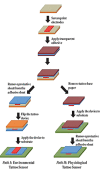
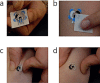
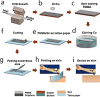
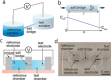

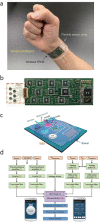
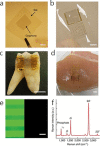


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

