The zebrafish: A fintastic model for hematopoietic development and disease
- PMID: 29436122
- PMCID: PMC6785202
- DOI: 10.1002/wdev.312
The zebrafish: A fintastic model for hematopoietic development and disease
Abstract
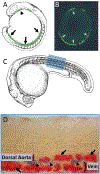
Hematopoiesis is a complex process with a variety of different signaling pathways influencing every step of blood cell formation from the earliest precursors to final differentiated blood cell types. Formation of blood cells is crucial for survival. Blood cells carry oxygen, promote organ development and protect organs in different pathological conditions. Hematopoietic stem and progenitor cells (HSPCs) are responsible for generating all adult differentiated blood cells. Defects in HSPCs or their downstream lineages can lead to anemia and other hematological disorders including leukemia. The zebrafish has recently emerged as a powerful vertebrate model system to study hematopoiesis. The developmental processes and molecular mechanisms involved in zebrafish hematopoiesis are conserved with higher vertebrates, and the genetic and experimental accessibility of the fish and the optical transparency of its embryos and larvae make it ideal for in vivo analysis of hematopoietic development. Defects in zebrafish hematopoiesis reliably phenocopy human blood disorders, making it a highly attractive model system to screen small molecules to design therapeutic strategies. In this review, we summarize the key developmental processes and molecular mechanisms of zebrafish hematopoiesis. We also discuss recent findings highlighting the strengths of zebrafish as a model system for drug discovery against hematopoietic disorders. This article is categorized under: Adult Stem Cells, Tissue Renewal, and Regeneration > Stem Cell Differentiation and Reversion Vertebrate Organogenesis > Musculoskeletal and Vascular Nervous System Development > Vertebrates: Regional Development Comparative Development and Evolution > Organ System Comparisons Between Species.
Keywords: blood; blood precursors; hemangioblasts; hematopoiesis; hematopoietic disorders; hematopoietic stem cells; leukemia; zebrafish.
Published 2018. This article is a U.S. Government work and is in the public domain in the USA.
Figures

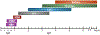
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

