Treatment Patterns and Associated Health Care Costs Before and After Treatment Initiation Among Pulmonary Arterial Hypertension Patients in the United States
- PMID: 29436260
- PMCID: PMC10398102
- DOI: 10.18553/jmcp.2018.17391
Treatment Patterns and Associated Health Care Costs Before and After Treatment Initiation Among Pulmonary Arterial Hypertension Patients in the United States
Abstract
Background: Despite multiple treatment options, the prognosis of pulmonary arterial hypertension (PAH) remains poor. PAH patients experience a high economic burden due to comorbidities, hospitalizations, and medication costs. Although combination therapy has been shown to reduce hospitalizations, the relationship between treatment, health care utilization, and costs remains unclear.
Objective: To provide a characterization of health care utilization and costs in real-world settings by comparing periods before and after initiating PAH-specific treatment.
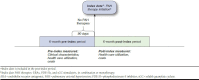
Methods: This retrospective study identified PAH patients in the Truven Health MarketScan Commercial and Medicare Supplemental Databases between 2010 and 2014 who initiated treatment with endothelin receptor antagonists (ERAs), phosphodiesterase-5 inhibitors (PDE-5Is), or soluble guanylate cyclase (sGC) stimulators. The index date was the date of the first PAH pharmacy claim. We included patients with ≥ 2 medical claims with diagnoses for PAH (ICD-9-CM: 416.0, 416.8) or PAH-related conditions and continuous enrollment in medical and pharmacy benefits for the 6 months before and after the index date. Treatment patterns were assessed at the drug class level (ERAs, PDE-5Is, sGC stimulators, and prostacyclins) from outpatient pharmacy claims during the 6-month post-index period. All-cause and PAH-related utilization and costs were measured. McNemar's and paired t-tests were used to compare patients' health care resource utilization and costs in the 6-month pre- and posttreatment periods.
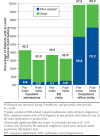
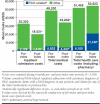
Results: A total of 3,908 patients met the selection criteria. The study sample was 63% female with a mean age of 63 ± 15 years. Only 5% of patients began initial combination therapy for PAH, defined as claims for ≥ 2 medication classes within the first 30 days of treatment. Treatment interruption (≥ 30-day gap in days supply) of any PAH-specific medication was observed in 38% of patients. Compared with the 6-month pre-index period, the proportion of patients in the 6-month post-index period with any inpatient admission decreased, 42% versus 30% (P < 0.001). In addition, PAH-related inpatient admissions decreased in the 6-month post-index period from 7% to 3% (P < 0.001). After treatment initiation, patients' nonpharmacy medical costs decreased from $48,200 (SD = $117,686) to $33,962 (SD = $90,294; P < 0.001), mainly attributable to reduced inpatient costs. However, total average medical costs including pharmacy costs remained comparable after treatment initiation (pre-index period = $51,455 vs. post-index period = $53,923; P = 0.213).
Conclusions: This study found that while patients' PAH-related pharmacy costs increased after treatment initiation, the increase was offset by reduced inpatient utilization; therefore, total health care costs remained constant. While the majority of patients in this study were treated with monotherapy, the recently completed AMBITION study indicated that initial combination therapy with ambrisentan plus tadalafil reduced PAH-related hospitalizations compared with initial monotherapy with either of these agents. Future cost analyses of patients treated with combination therapy will be required to determine the economic effect of initial combination therapy.
Disclosures: This study was sponsored and funded by Gilead Sciences. Ozbay is an employee of Gilead Sciences. At the time that this project and manuscript were developed, Lazarus was an employee of Gilead Sciences and may own stock/stock options. Riehle, Montejano, and Lenhart are employees of Truven Health Analytics, an IBM company, which received funding from Gilead Sciences to conduct this study. Burger and White do research with, and are paid consultants for, Gilead Sciences; they do not own equity and received no personal compensation for the work here. Burger also reports consultancy and advisory board work for Actelion Pharmaceuticals and grants from Gilead Sciences, Actelion Pharmaceuticals, Bayer, and United Therapeutics.
Conflict of interest statement
This study was sponsored and funded by Gilead Sciences. Ozbay is an employee of Gilead Sciences. At the time that this project and manuscript were developed, Lazarus was an employee of Gilead Sciences and may own stock/stock options. Riehle, Montejano, and Lenhart are employees of Truven Health Analytics, an IBM company, which received funding from Gilead Sciences to conduct this study. Burger and White do research with, and are paid consultants for, Gilead Sciences; they do not own equity and received no personal compensation for the work here. Burger also reports consultancy and advisory board work for Actelion Pharmaceuticals and grants from Gilead Sciences, Actelion Pharmaceuticals, Bayer, and United Therapeutics.
Figures
Similar articles
-
Prostacyclin Use Among Patients with Pulmonary Arterial Hypertension in the United States: A Retrospective Analysis of a Large Health Care Claims Database.J Manag Care Spec Pharm. 2018 Mar;24(3):291-302. doi: 10.18553/jmcp.2017.17228. Epub 2017 Dec 18. J Manag Care Spec Pharm. 2018. PMID: 29406840 Free PMC article.
-
Real-World Economic Outcomes During Time on Treatment Among Patients Who Initiated Sunitinib or Pazopanib as First Targeted Therapy for Advanced Renal Cell Carcinoma: A Retrospective Analysis of Medicare Claims Data.J Manag Care Spec Pharm. 2018 Jun;24(6):525-533. doi: 10.18553/jmcp.2018.24.6.525. J Manag Care Spec Pharm. 2018. PMID: 29799328 Free PMC article.
-
Treatment Patterns and Medication Use in Patients with Postherpetic Neuralgia.J Manag Care Spec Pharm. 2019 Dec;25(12):1387-1396. doi: 10.18553/jmcp.2019.19093. Epub 2019 Oct 7. J Manag Care Spec Pharm. 2019. PMID: 31589557 Free PMC article.
-
A Review of Clinical Trial Endpoints of Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension and How They Relate to Patient Outcomes in the United States.J Manag Care Spec Pharm. 2017 Jan;23(1):92-104. doi: 10.18553/jmcp.2017.23.1.92. J Manag Care Spec Pharm. 2017. PMID: 28025931 Free PMC article. Review.
-
Estimating HIV Management and Comorbidity Costs Among Aging HIV Patients in the United States: A Systematic Review.J Manag Care Spec Pharm. 2020 Feb;26(2):104-116. doi: 10.18553/jmcp.2020.26.2.104. J Manag Care Spec Pharm. 2020. PMID: 32011956 Free PMC article.
Cited by
-
Real-World Analysis of Treatment Patterns Among Hospitalized Patients with Pulmonary Arterial Hypertension.Pulm Ther. 2021 Dec;7(2):575-590. doi: 10.1007/s41030-021-00173-6. Epub 2021 Oct 26. Pulm Ther. 2021. PMID: 34699029 Free PMC article.
-
Accuracy of Algorithms to Identify Pulmonary Arterial Hypertension in Administrative Data: A Systematic Review.Chest. 2019 Apr;155(4):680-688. doi: 10.1016/j.chest.2018.11.004. Epub 2018 Nov 22. Chest. 2019. PMID: 30471268 Free PMC article.
-
Societal Costs Associated With Pulmonary Arterial Hypertension Subgroups: A Study Utilizing Linked National Registries.Pulm Circ. 2025 Apr 17;15(2):e70074. doi: 10.1002/pul2.70074. eCollection 2025 Apr. Pulm Circ. 2025. PMID: 40248212 Free PMC article.
-
Treatment patterns, healthcare resource utilization, and healthcare costs among patients with pulmonary arterial hypertension in a real-world US database.Pulm Circ. 2019 Jan-Mar;9(1):2045894018816294. doi: 10.1177/2045894018816294. Epub 2018 Nov 13. Pulm Circ. 2019. PMID: 30421652 Free PMC article.
-
Societal costs associated with pulmonary arterial hypertension: A study utilizing linked national registries.Pulm Circ. 2023 Jan 1;13(1):e12190. doi: 10.1002/pul2.12190. eCollection 2023 Jan. Pulm Circ. 2023. PMID: 36704610 Free PMC article.
References
-
- Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119. - PubMed
-
- Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2015;46(4):903-75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous