Subcutaneous treprostinil in congenital heart disease-related pulmonary arterial hypertension
- PMID: 29436381
- PMCID: PMC6047165
- DOI: 10.1136/heartjnl-2017-312143
Subcutaneous treprostinil in congenital heart disease-related pulmonary arterial hypertension
Abstract
Objective: To assess the efficacy and safety of subcutaneous treprostinil in adult patients with congenital heart disease (CHD)-associated pulmonary arterial hypertension (PAH) after 12 months of treatment.
Methods: Consecutive adult patients with CHD-PAH received subcutaneous treprostinil to maximum tolerated doses in an observational study.
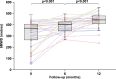
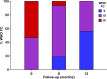
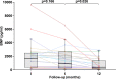
Results: Advanced CHD-PAH patients with WHO class III or IV disease (n=32, age 40±10 years, 20 females) received treprostinil for suboptimal response to bosentan (n=12), WHO functional class IV disease (FC, n=7) or prior to bosentan approval (n=13). In the multivariate mixed model, mean increase in 6 min walk distance (6-MWD) from baseline to 12 months was 114 m (76; 152) (P<0.001). WHO FC improved significantly (P=0.001) and B-type brain natriuretic peptide decreased from 1259 (375; 2368) pg/mL to 380 (144; 1468) pg/mL (P=0.02). In those 14 patients who had haemodynamic data before and after initiation of treprostinil, pulmonary vascular resistance decreased significantly (from 18.4±11.1 to 12.6±7.9 Wood units, P=0.003). The most common adverse events were infusion-site erythema and pain. One patient stopped treatment because of intolerable infusion-site pain after 8 months of treatment. No other major treatment-related complications were observed. Five patients died during early follow-up, having experienced a decrease in their 6-MWD prior.
Conclusions: Subcutaneous treprostinil therapy is generally safe and effective for at least 12 months and may be used in CHD-related PAH class III and IV.
Keywords: secondary pulmonary hypertension.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: NS-S reports personal fees from GlaxoSmithKline, grants and personal fees from AOPOrphan Pharmaceuticals, grants and personal fees from Actelion, personal fees from Bayer AG, personal fees from Pfizer, personal fees from United Therapeutics, outside the submitted work.
Figures
References
-
- Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. 10.1093/eurheartj/ehv317 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
